MADES - Integrated Electronic Manufacturing Services
MADES has been engaged in industrialising, manufacturing, testing and integrating high-reliability electronic devices for critical medical applications for more than 30 years.

MADES provides industrialisation, manufacturing, testing and integration services for medical device electronics.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

MADES has been providing services for the industrialisation, manufacture, testing and integration of high-reliability electronic devices for critical medical applications since 1990.
Our vision is to be the world’s leading supplier of precision electronics and exceed clients’ highest expectations while complying with all regulatory requirements.
We want to assure our clients that their orders will be implemented using the most sophisticated manufacturing processes and advanced technology available, by passionate experts with highly stringent management systems.
MADES is involved in several international projects in the medical device sector, which require high-reliability manufacturing and assembly.
In the industrialisation phase, we are involved in design activities, providing advice for clients and guiding them towards operational excellence in the manufacturing stage.
Our team of engineers designs the flow of processes and controls to optimise cost and quality. Our testing strategy includes equipment development and can be adapted to each type of product and client.
As part of integration activities, MADES also offers process control, highly complex parametric and functional tests, and specific environmental tests to guarantee the required level of reliability for each project.
MADES uses a Six Sigma programme to achieve ever-higher levels of value for its clients. We employ these principles with the aim of attaining perfection. They form the basis of our corporate culture: mission assurance.
Our company has built its reputation by creating the most demanding high-precision electronic systems. We aim to ensure that the end-user can trust the electronics in their products to work perfectly at critical moments.
Our clients rely on us to do our job properly so that they can do theirs, so our corporate culture is their quality assurance.
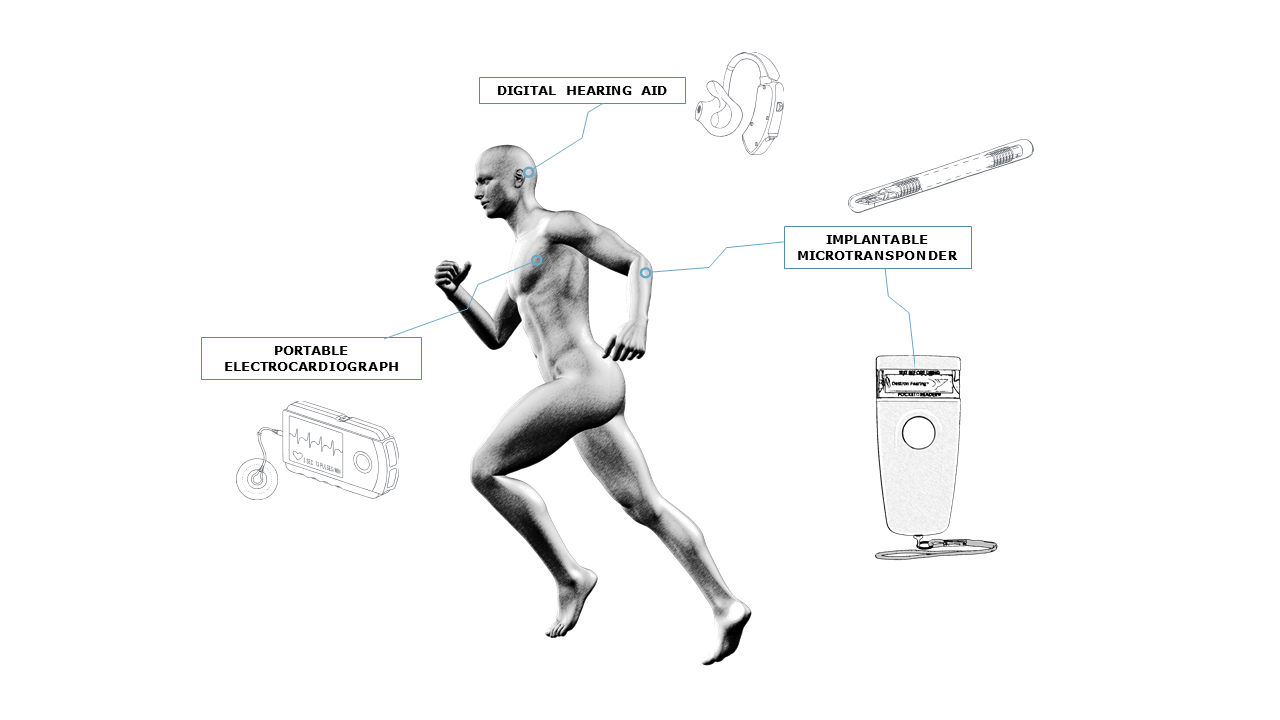
The medical industry requires highly precise electronic manufacturing services that guarantee the safety and effectiveness needed to meet each country’s regulatory standards.
By working with MADES engineers, our clients can guarantee excellence in mechanical and electronic design from the outset. Using our engineering experience and the possibilities it presents, we can shorten the programme cycle and produce more cost-effective and reliable products as and when clients need them.
Our engineers work on research and development (R&D) projects to identify effective solutions for the industry in terms of cost and production process efficiency, with the aim of meeting all our clients’ needs and expectations.
MADES offers cleanroom manufacturing space and more than 30 years of experience and technological knowledge in the medical device manufacturing sector.
We collaborate with our clients to ensure compliance with the strict regulatory requirements of the medical device industry, including those established by the US Food and Drug Administration (FDA) and outlined in the International Organisation for Standardisation (ISO) 13485 certification.
Quality certifications held by MADES include ISO 9001, Nadcap 7120 Electronics, Nato Allied Quality Assurance Publications (AQAP) 2110, and EN9100 Aerospace and Defense. Our manufacturing processes and quality system are also aligned with ISO 13485 requirements.

MADES has been engaged in industrialising, manufacturing, testing and integrating high-reliability electronic devices for critical medical applications for more than 30 years.

MADES is a former subsidiary of Hughes Aircraft & Raytheon Missile Systems located in Malaga, Spain. We have been manufacturing circuit card assemblies and electronic systems for a range of international weapon systems and platforms since 1990.

MADES supports technological development and the innovation ecosystem by partnering with a wide range of companies and projects.
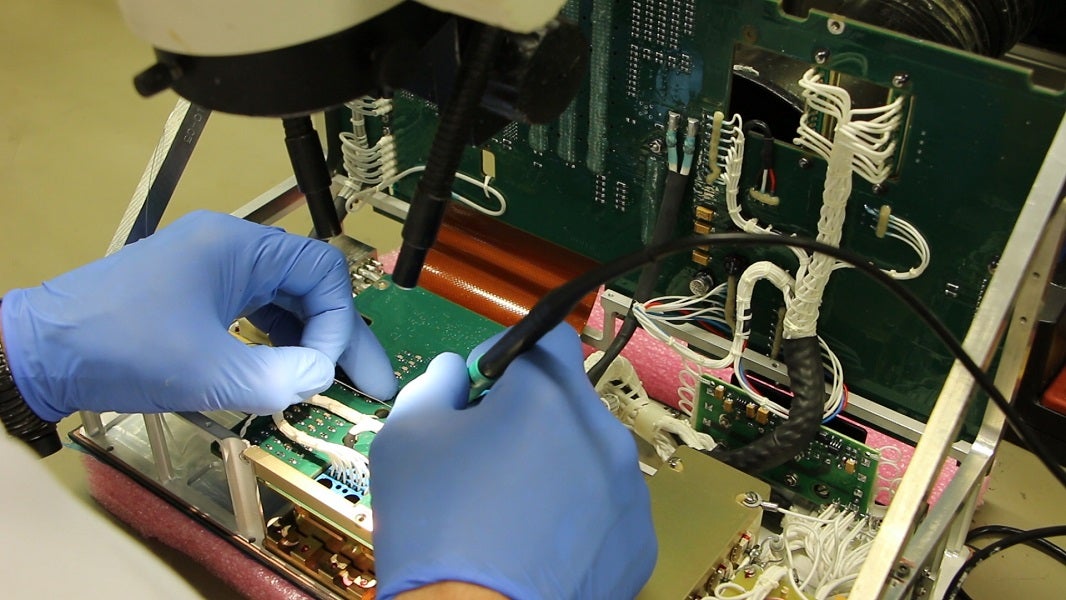
The design and manufacture of complex electronic systems has been one of MADES’ core capabilities for over 25 years.

MADES utilizes a full suite of computerized optical, electronic, functional and environmental tests for qualifying new designs and ensure consistent quality.
MADES seamlessly integrates its advanced electronics capabilities to final turn-key customer solutions.
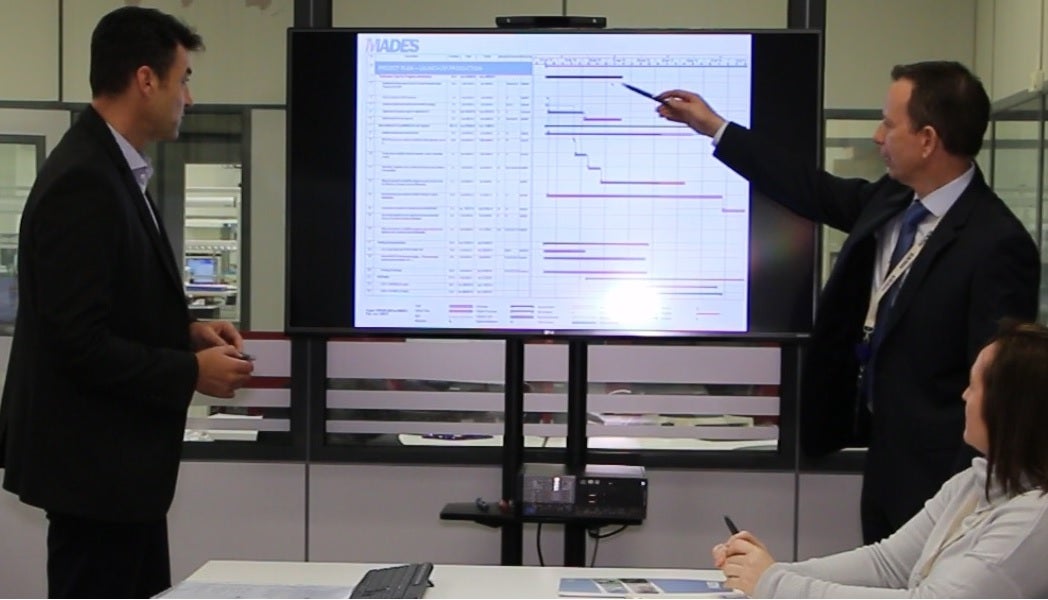
MADES offers services such as design for manufacturing, industrialisation, obsolescence management, strategic purchasing and the redesign of obsolete products.

MADES is engaged in industrialising, manufacturing, testing and integrating highly reliable electronic devices for critical medical applications.