Germany-based medical technology company B. Braun has won two awards in the 2024 Medical Device Excellence Awards, in the Product Launches and Safety categories, for launching the Introcan Safety® 2 IV Catheter, which is a significant advancement in vascular access devices.
The comprehensive safety features of Introcan Safety® 2, coupled with its efficiency-enhancing design, have won B. Braun the recognition.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Medical Device Excellence Awards celebrate the greatest achievements and innovations in the industry. Powered by the business intelligence of GlobalData, the programme provides a platform to recognise the people and companies that are driving change in the industry.
Launched in 2023, the Introcan Safety® 2 IV Catheter represents a leap forward in ensuring the safety of both patients and healthcare professionals during vascular access procedures. This short safety peripheral intravenous catheter, designed for short-term use, is a versatile tool for blood sampling, blood pressure monitoring, and the administration of fluids and blood intravascularly. Its suitability for subcutaneous infusion therapies further extends its utility across various clinical scenarios.
Design and functionality: Enhancing clinical efficiency
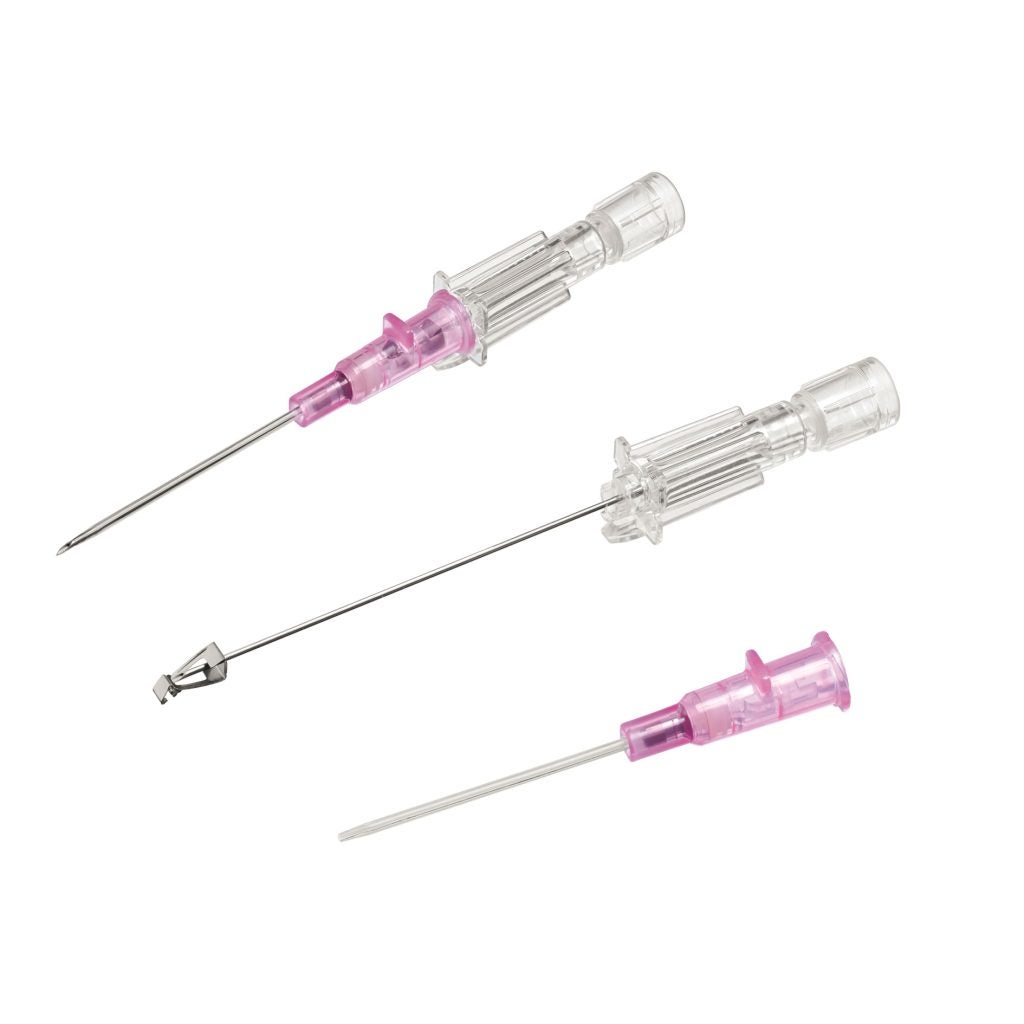
The Introcan Safety® 2’s multi-access blood control septum is a distinctive feature that distinguishes it from traditional catheters. It plays a critical role in minimising blood exposure during catheter insertion and disconnection events.
By significantly reducing the need for venous compression, it not only enhances the safety profile of the device but also contributes to a cleaner and more controlled working environment. This reduction in blood exposure is a significant advancement, as it decreases the likelihood of contact with potentially infectious material, thereby safeguarding both medical personnel and patients.
Advancing patient and clinician safety
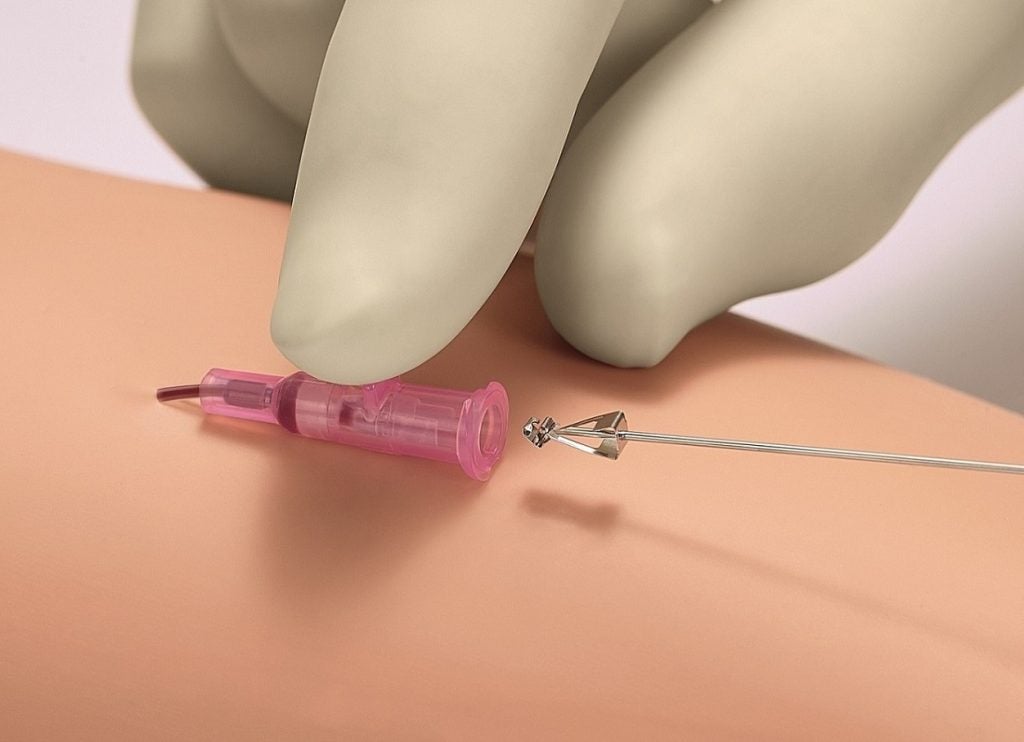
Central to the Introcan Safety® 2’s design is the passive safety shield. This fully-automatic feature is a pivotal innovation in the prevention of needlestick injuries, which are a significant concern in medical settings. The shield deploys automatically upon needle withdrawal, requiring no active engagement from the user, thus ensuring consistent protection that cannot be bypassed.
By significantly reducing the risk of needlestick injuries, the Introcan Safety® 2 also minimises the potential for subsequent infections, which can be caused by accidental exposure to bloodborne pathogens. This safety feature is particularly crucial in high-risk environments and contributes to a safer workplace, aligning with the latest health and safety protocols. The importance of this safety mechanism cannot be overstated, as needlestick injuries are not only a health hazard but also carry psychological and emotional stress for medical staff.
By providing an additional layer of safety, B. Braun demonstrates its commitment to the well-being of healthcare professionals, which in turn can lead to a more focused and confident approach to patient care.
Patient comfort and environmental considerations
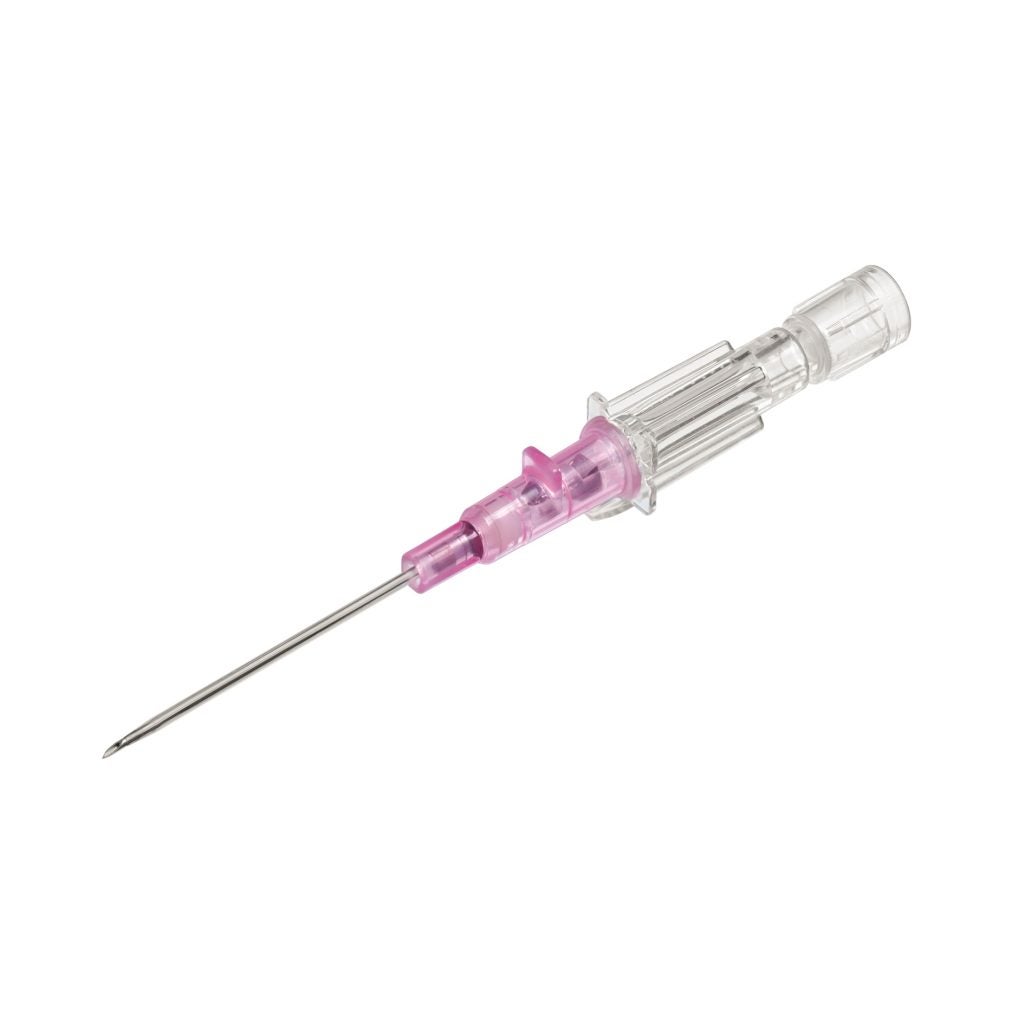
In terms of functionality, the Introcan Safety® 2 is power injectable, accommodating the use of power injectors up to a maximum pressure of 325psi. This feature ensures compatibility with modern medical imaging techniques, where rapid administration of contrast media is often required.
The catheter’s universal back cut bevel offers clinicians a wide choice of insertion angles, catering to various patient anatomies and clinical situations. This design consideration aims to minimise puncture trauma, enhancing patient comfort during the insertion process.
Fruthermore, the double flashback technology is another key advantage, providing visual confirmation that both the needle and the catheter are correctly placed within the vessel.
The awards for Introcan Safety® 2, underscore B. Braun’s role in setting new standards for patient care and clinician safety in the medical technology sector.
“Winning the award for Introcan Safety® 2 is a recognition of the entire product cycle – from development to approval and marketing. I would therefore like to thank all B. Braun employees who have contributed to this success with their expertise.”
Yongji Fu, Vice President & Head of R&D
Company Profile

As one of the world’s leading medical technology companies, B. Braun protects and improves the health of people around the world. For over 180 years, the family-owned company has been accelerating progress in healthcare with pioneering spirit and groundbreaking contributions. This innovative strength continues to be the foundation of B. Braun’s success today – always with the goal of improving clinical outcomes, cost of care and patient benefits.
The expertise shared by more than 65,000 employees worldwide makes B. Braun a true partner that develops smart solutions and sets new standards. By linking products, services and consulting, the company improves treatment processes and supports medical staff. In doing so, B. Braun always acts with future generations in mind, which is why responsibility for sustainable growth is embedded into all business processes. In 2022, the B. Braun Group generated sales of €8.5bn.
Contact Details
B.Braun Melsungen AG
Carl-Braun-Straße 1
34212 Melsungen, Hessen,
Germany
Phone: +49 5661 710
Links
Website: https://bbraun.com
Introcan Safety® 2: Introcan Safety® 2 | B. Braun (bbraun.com)





