
Abbott closes acquisition of Idev Technologies for $310m
US-based Abbott has completed the acquisition of Idev Technologies, a Texas-based company focused on developing next-generation medical devices, for $310m in a mixed net cash and debt deal.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The deal, which was announced in July 2013, was initially expected to closed by the end of the year following regulatory clearances.
The acquisition of Idev Technologies will expand and complement Abbott’s existing peripheral technology portfolio of guidewires, balloon dilatation catheters and stents.
Kinetic Concepts to acquire wound care products maker Systagenix
Kinetic Concepts (KCI), a US-based medical technology provider, has signed a definitive agreement to acquire Systagenix, a UK-based advanced wound care (AWC) products maker, for $485m as part of its strategy to boost revenues and expand its geographic presence.
The deal is also expected to improve KCI’s position in the international market. With a wide portfolio of AWC products and presence in categories of foam and contact layers, Systagenix distributes more than 20 million advanced wound care dressings each month to more than 100 countries worldwide.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSeveral of Systagenix’s products are used before, during and after use of KCI’s core negative pressure wound therapy products.
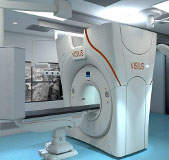
IMRIS receives CE mark for intraoperative computed tomography

IMRIS, an image-guided therapy solutions provider, has received CE mark for its ceiling-mounted VISIUS intraoperative computed tomography (iCT).
The CE mark will allow the company to sell and market the product in the European Union.
VISIUS iCT helps surgeons in making critical decisions by providing personalised dose management and diagnostic quality imaging.
St Jude Medical to buy Swiss medical device maker Endosense
US-based medical device maker St Jude Medical has agreed to acquire Endosense, a Switzerland-based medical technology company that works in the development of catheter ablation for cardiac arrhythmia treatment, in a $331m deal.
With an initial payment of $170m (CHF159m), the terms of the transaction include an additional payment of up to $161m (CHF150m) in cash, following the regulatory approvals within a designated timeframe.
St. Jude Medical cardiovascular and ablation technologies division president Frank J Callaghan noted that the acquisition of Endosense will further strengthen the company’s industry-leading portfolio of products to treat patients with cardiac arrhythmias, and provide an opportunity to accelerate market share capture in the $900m global cardiac ablation catheter market.
LDR secures first FDA clearance for two-level cervical disc replacement

LDR has obtained approval from the US Food and Drug Administration (FDA) for commercial sale and distribution of its Mobi-C Cervical Disc (Mobi-C) for two-level indications in the US.
Earlier this month, the company has secured a clearance from the FDA for commercial sale and distribution of the Mobi-C for one-level use.
The FDA has approved the Mobi-C to be used as a safe and effective surgical option for both one and two-level indications following positive results from the multi-centre clinical trials.
C R Bard to acquire Medafor for $200m
C R Bard has agreed to acquire Medafor, a developer and supplier of plant-based hemostatic agents, for a purchase price of $200m.
The deal, which is expected to close later in 2013, includes contingent payments of up to an additional $80m, based on achieving specific revenue-based milestones through to 30 June, 2015.
The transaction which has been approved by both companies’ boards of directors is subject to an approval by Medafor’s shareholders and customary regulatory review.
Given Imaging’s PillCam SB 3 video capsule receives 510(k) clearance

Israel-based medical devices developer Given Imaging has got clearance from the US regulatory agency to market its next-generation video capsule PillCam SB 3, which can detect small bowel abnormalities related to Crohn’s disease and gastrointestinal bleeding.
Weighing less than 4g, the 11mm by 26mm video capsule features an imaging device and a light source that can transmit anywhere between two and six images in one second.
Due to software enhancements, the PillCam SB 3 offers a 40% more efficient video compilation than the earlier SB 2 version. The SB 3 will be available in the US towards the end of the year.
FDA grants 510(k) clearance for Zimmer PSI Shoulder system
The US Food and Drug Administration (FDA) has granted 510(k) clearance to the Zimmer Patient Specific Instruments (PSI) shoulder system.
The PSI shoulder system helps surgeons develop a customised surgical plan for each patient by using 3D visualisation software. It complements Zimmer’s Trabecular Metal Reverse base plate implant system, designed for reverse shoulder arthroplasty (RSA) procedures.
The system also provides patient-specific surgical instrument guides to facilitate placement of the implant corresponding to the personalised surgical plan.
By using these plans, surgeons can size and position the implant for placement, and can even prepare bones and position screws for implant placement.





