A number of leading and emerging technologies are having a revolutionary effect on the device-based diagnosis and treatment landscape. Here we invesigate ten more, following on from our earlier look at ten top tips.
Ultra-high field magnetic resonance imaging

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
As clinical applications continue to demand higher resolution images, use of ultra-high field magnetic resonance imaging (MRI) technology will become inevitable. While 3T MRI has become clinical reality, clinical investigations are underway to test 7T MRI and 11.7T MRI systems for human applications.
The 7T MRI system, while offering the potential for microscopic spatial resolution, also enables the observation and analysis of tissue metabolism and function. Being developed by Siemens Healthcare, at the moment the 7T MRI system is an investigational research device and is not available for clinical use.
It is expected that the 7T MRI system will make it possible to study neuronal function at the sub-millimetre scale. Potential clinical applications of this system include neurodegenerative diseases like Alzheimer.
The 11.7T MRI system, being developed by the French Atomic Energy Commission in collaboration with Siemens Healthcare and Alstom Magnets and Superconductors, within the framework of the French-German consortium Iseult/INUMAC (Imaging of Neuro disease Using high field MAgnetic resonance and Contrastophores) is expected to address clinical applications of ultra-high field MRI.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataContinuous glucose monitoring
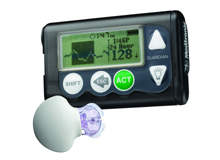
The majority of insulin-dependant diabetics test their blood glucose levels three times a day to make sure that their blood glucose is within a clinically acceptable range. However, there are often periods of hyperglycaemia or hypoglycaemia that are short-lived but go undetected. These unmeasured instances increase the risks of complications. Continuous glucose monitoring (CGM) addresses this problem by allowing improved glycaemic control; by decreasing the instances of hyperglycaemic or hypoglycaemic stages and by increasing the time spent in the acceptable range.
Patients who use CGM are informed about their blood glucose levels on a 24/7 basis with test readings as often as every five minutes. This means that CGM systems can provide up to 288 readings a day against the standalone readings that the finger-stick-based conventional self monitoring blood glucose systems provide. It is believed that CGM systems have the potential to revolutionise the way diabetes is being managed and treated. With more accurate and relatively real-time data at their disposal, physicians have the capability to develop a personalised disease management regime for each patient.
Kyphoplasty
Kyphoplasty is an alternative procedure to treat vertebral compression fractures (VCF). The goal of kyphoplasty is to correct the deformity and stabilise the spine in a patient with a VCF. A large proportion of these fractures are caused by osteoporosis, primarily found in older people whose bones have been weakened.
Kyphoplasty is an innovative surgical procedure that enables the surgeon to access the spine with two small cannulae and deliver orthopaedic balloons to the centre of the collapsed bone.
The balloons are inflated, which compresses the inner bone and pushes the outer bones apart to restore anatomy. Afterwards, the balloons are removed and filler is inserted to stabilise the bone.
Kyphoplasty is preferred in cases of severe collapse of the broken vertebra or wedging with more damage in the front of the spine. It also helps to restore the spine to a more normal height and prevent severe kyphotic deformity.
In patients with multiple fractures with previous wedging, kyphoplasty can prevent further worsening of the deformity.
In the last eight years, 500,000 kyphoplasty procedures have been carried out across the globe. The US remains the key market, followed by Europe. The Asian market remains relatively undertapped, with most of the product sales in this area coming from Japan, China and India.
Capsule endoscopy
While capsule endoscopy (CE) has been on the market since 2001, its role in managing obscure gastrointestinal bleeding and many other small bowel diseases continues. Wireless capsule endoscopy is not only non-invasive but also is a safe, simple and reliable procedure, well accepted and tolerated by patients. The capsule endoscope is a wireless miniature camera that is disposable and can be swallowed, allowing visualisation of the whole small bowel by producing high-resolution images.
The first capsule endoscope was developed by Israel’s Given Imaging and approved in Europe by the European Medicines Agency and in the US by the Food and Drug Administration in 2001. GlobalData estimates that the market for capsule endoscope systems, valued at $163.5m in 2008, is forecast to grow by an average 7% annually during the next seven years to reach $261.3m by 2015.
Nucleus replacement
Nucleus replacement is an alternative to the total replacement of an intervertebral disc in the spinal cord. Indicated for early-stage degenerative disc disease (DDD), the purpose of a nucleus replacement procedure is to relieve pain and restore the mechanical properties assured by a healthy intervertebral disc. In a nucleus replacement procedure, the jelly-like material of the intervertebral disc is replaced with an artificial biocompatible material.
Nucleus replacement offers a less invasive alternative to traditional fusion or total disc replacement techniques in the treatment of symptomatic lumbar DDD. The procedure is less invasive, less traumatic and most importantly, motion preserving, which is expected to drive acceptance and market penetration of nucleus replacement products.
Atrial fibrillation
Atrial fibrillation is one of the most common irregular heart rhythms and it reduces the heart pumping by 25–30%. when compared with electrical stimulation devices like implantable cardioverter defibrillators or pacemakers.
Radio-frequency catheter ablation is the only permanent treatment available for patients suffering from certain types of abnormal fast heartbeats, like atrial flutter, atrial fibrillation, atrial tachycardia, atrioventricular nodal reentrant tachycardia, and atrioventricular reentrant tachycardia. Catheter ablation for atrial fibrillation
A catheter ablation technique to treat atrial fibrillation involves a catheter inserted through the groin area into the heart. A special machine delivers energy through the catheter to tiny areas of the heart muscle that cause the abnormal heart rhythm. Cells responsible for inappropriate conduction of electricity in the heart are ablated using radio frequency energy. The ablation procedure disconnects the electrical pathway between the upper chambers (atria) and lower chambers (ventricles) of the heart. The type of ablation performed depends on the type of arrhythmia.
Biosense Webster, a Johnson & Johnson company, became the first to gain the US FDA’s marketing approval for an ablation catheter for treatment of heart rhythm disorder, when its Navistar Thermocool catheter was approved in early 2009.
Point-of-care testing
Point-of-care testing (POCT) is defined as medical diagnostic testing at or near the site of patient care – the objective being an immediacy of response. While the intensive care unit and the patient’s home environment remain the most common sites for the use of POCT devices, advances in technology have resulted in the devices being used in many other delivery settings.
POCT enables clinicians to monitor in real time and at the site of care, the vital health signs of patients – the result being early recognition of life-threatening conditions.
The fact that these devices and kits do not require dedicated space and can be performed outside the physical facilities of clinical diagnostic laboratories is another factor contributing to the adoption and market penetration of these products. POCT products bring test facilities conveniently and immediately to the site of care, increasing the likelihood that immediate clinical action will be undertaken, should the need arise.
POCT is being increasingly used to address clinical needs during life-threatening cardiac arrests and strokes. POCT is increasing test turnaround time, enabling clinicians to earlier diagnose and address life-threatening conditions.
Most importantly, POCT has reduced turnaround time, improved clinicians’ satisfaction and has boosted the bottom line of hospitals by minimising unnecessary load on their existing infrastructures.
Needle-free injections
Needle-free drug delivery systems use a liquid drug delivery technology that is designed to work with existing drug formulations. These systems have been successful in addressing challenges posed by traditional drug delivery methods such as overcoming a patient’s aversion towards needles, elimination of infection risk, painless delivery of drugs and ease of use. New formulations are designed to gradually release the injected drug, reducing the frequency of injections.
Needle-free injection systems that also ensure precise dosing and reduce risks of cross-contamination are widely being used for intradermal administration of drugs, vaccines, hormones and anaesthetics. GlobalData estimates that the global needle-free injections market, valued at $572 million in 2008, is forecast to grow by over 75% annually for the next seven years to reach $29bn by 2015.
Digital breast tomosynthesis
The digital breast tomosynthesis (DBT) mammography system acquires, processes and visualises data from 3D X-ray images making it easier to detect tumours even in dense breast tissue. DBT reconstructs a 3D composite image from a series of 11 low-dose 2D images, enabling physicians and radiologists to view the interior of the breast without obstruction from surrounding superimposed breast tissues.
The system enables physicians to identify tumours that are easy to miss with the conventional 2D approach. Under clinical investigation, once approved the system is expected to redefine the breast cancer diagnosis and treatment landscape.
DBT-based mammography systems are being developed independently by GE Healthcare, Siemens Healthcare, US firm Hologic, Inc and the UK’s Dexela Limited.
SPECT/CT
SPECT/CT technology remains the youngest hybrid molecular imaging technology in the imaging industry. SPECT/CT systems not only offer additional clinical applications but also can be used as multipurpose cameras, often serving the needs of radiology and nuclear medicine imaging departments.
While these systems have been in the market for a while, the fact that they serve some advanced diagnostic needs will continue to drive the demand and acceptance of this technology. The diagnostic imaging big-three – GE Healthcare, Siemens Healthcare and Philips Healthcare – all have SPECT/CT systems in their existing imaging portfolios.





