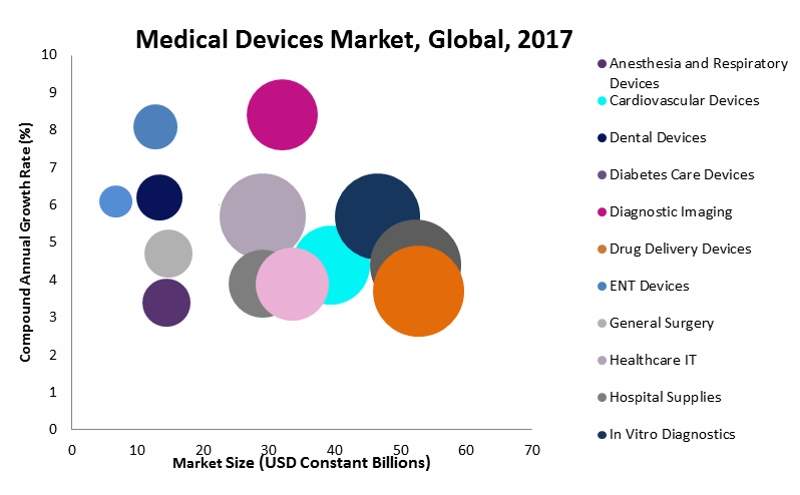
 The global medical devices market was estimated to be worth more than $450bn with a compound annual growth rate (CAGR) of 3.9% in 2017. Competition is fierce, with each company fighting to expand their share of the market.
The global medical devices market was estimated to be worth more than $450bn with a compound annual growth rate (CAGR) of 3.9% in 2017. Competition is fierce, with each company fighting to expand their share of the market.
Quick releases of cutting-edge products can maximise profits, but a recent study published in the Journal of the American Medical Association has shown that it can also threaten patient safety.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the UC San Francisco study, researchers found that a large number of high-risk and life-support devices are rushed through the US Food and Drug Administration (FDA) approval process, and many clinical studies of these devices are haphazardly designed. Some of these studies lack basic experimental controls, last less than six months, or fail to present data from all participants. The FDA approval process for next-generation devices was found to be even more lax than the approval process for newly developed products.
There are few protections for patients harmed by FDA approved devices, even if the approval process was found to be rushed. The 2008 court case Riegel vs Medtronic set the precedent for FDA approval protecting manufacturers from lawsuits over device safety, even if approval was based on a poorly conducted study.
Although product recalls can be costly, causing up to $5bn in losses a year for the global industry, profits reaped from aggressively pushing to market make it worth the risk. The number of medical device recalls per year has nearly doubled in the past decade.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData




