Applied DNA Sciences’ clinical laboratory subsidiary, Applied DNA Clinical Labs, has submitted a request seeking Emergency Use Authorisation (EUA) from the US Food and Drug Administration (FDA) for two products.
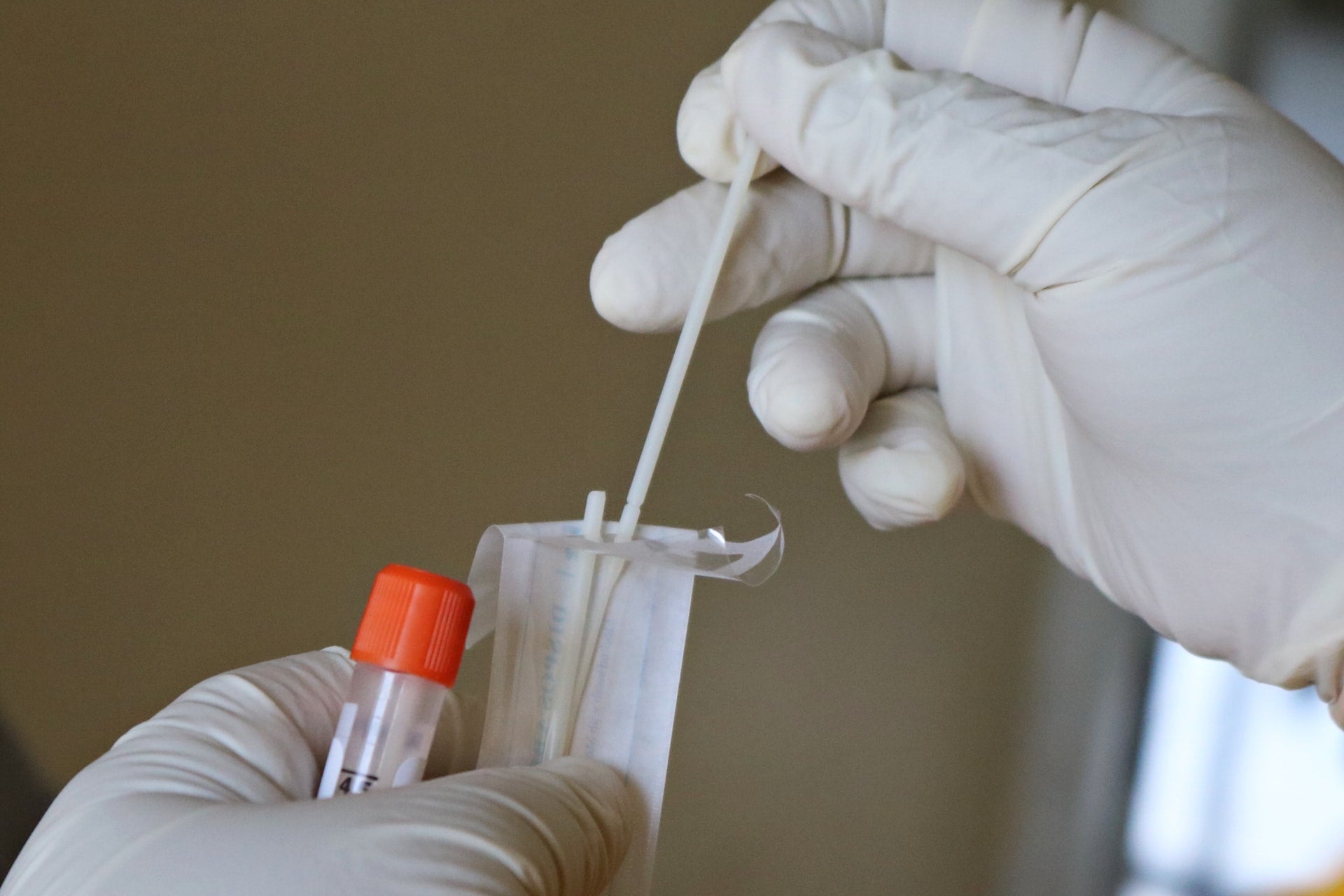
These products are the Linea 2.0 COVID-19 Assay and Linea Unsupervised At-Home Sample Collection Kit.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Linea 2.0 Assay is a multiplex RT-PCR assay designed to target the E and N genes of SARS-CoV-2. It can detect all known SARS-CoV-2 variants.
The company stated that the assay is validated for single-sample and robotic-pooled testing.
Last December, the New York State Department of Health granted conditional approval to the Linea 2.0 COVID-19 Assay.
The Linea At-Home Sample Collection Kit allows simple self-collection of nasal swab samples without medical personnel supervision.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe individual samples can be sent directly back to the company or collected by a testing client and shipped back to the company in bulk.
Results are usually returned within 24 to 48 hours of a sample’s arrival at the company’s clinical lab.
The company stated that the EUA approval will allow it to expand its safeCircle Covid-19 testing platform across the nation.
The fully integrated testing platform provides Covid-19 diagnostic testing as well as other services, including sample collection, results tracking, vaccination status management, and test site infrastructure design and management, for enterprise and educational institutions.
Applied DNA president and CEO Dr James Hayward said: “We believe this EUA request positions us to service existing demand for enterprise-scale Covid-19 testing with a compelling selling proposition where remote work is not a scalable option and a dependence on less sensitive, antigen-based tests can potentially lead to outbreaks and interruptions in business continuity.
“At the same time, while safeCircle was formed specifically to build a Covid-19 testing platform, the potential acquisition of a national customer base advances ADCL’s strategy to expand its diagnostic offerings beyond SARS-CoV-2 with an installed client base.”
Last January, the FDA published a safety communication that identified Applied DNA Sciences’ Linea COVID-19 Assay Kit as one of only two tests marketed under its EUA for the detection of certain mutations of SARS-CoV-2.





