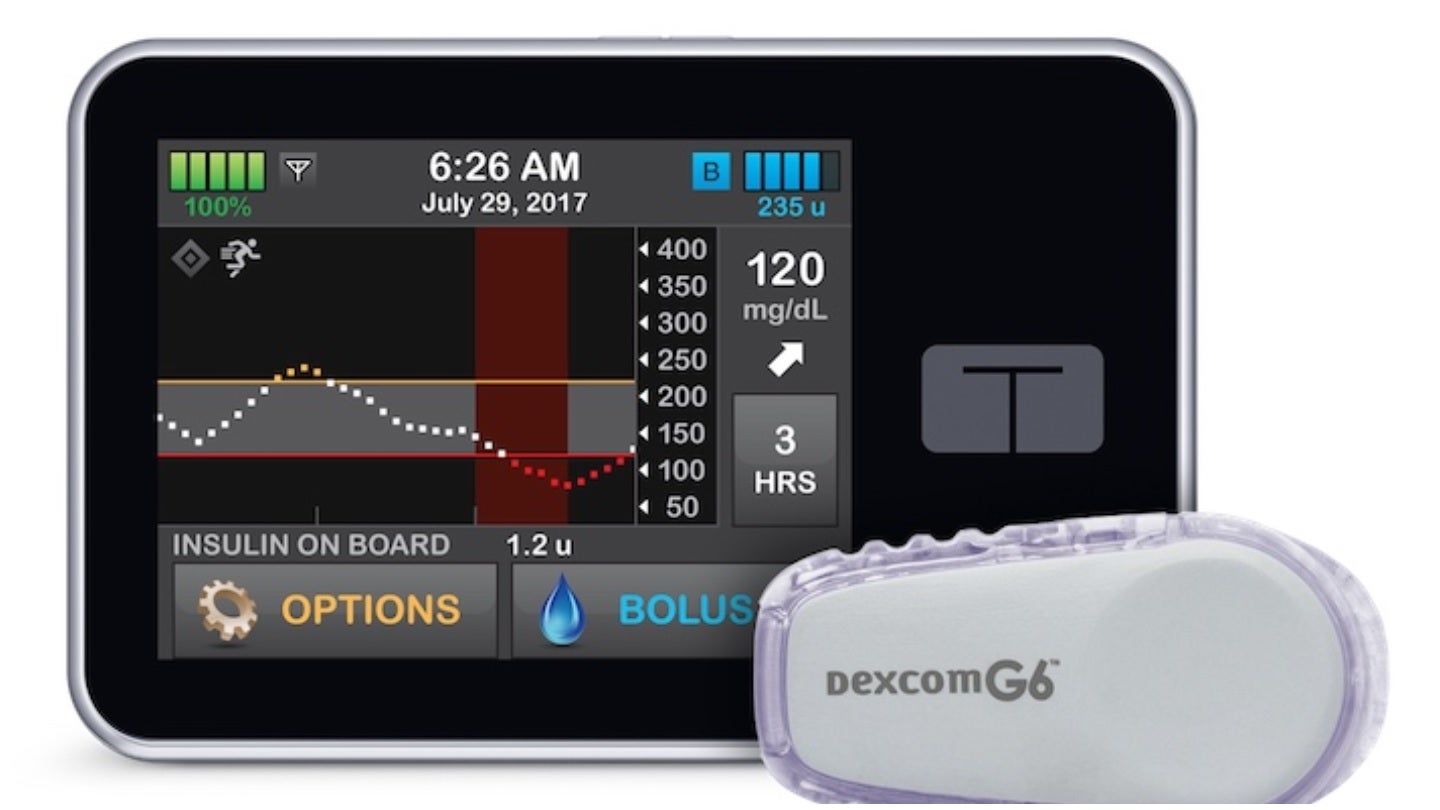
A new combined analysis of three clinical trials has revealed that an artificial pancreas developed at the University of Virginia Center for Diabetes Technology improves blood sugar control for people aged between two and 72 with type 1 diabetes.
The artificial pancreas is manufactured by Tandem Diabetes Care and marketed as the Control-IQ system.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The study found an average of 2.8 additional hours a day of controlled blood sugar within the target range in participants using the artificial pancreas across the three trials, compared with participants in control groups who received standard treatment to manage their blood sugar.
The new analysis was based on results from 369 people who participated in trials at eight US sites, including UVA Health.
The team assigned 256 participants to use the artificial pancreas system while the remaining 113 were assigned to a control group.
As per the analysis, the average time spent by participants who received the artificial pancreas within their target blood glucose range was 13 percentage points higher compared to participants in the control group.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFurthermore, the findings revealed significantly reduced haemoglobin A1c (average blood sugar) levels in patients with the artificial pancreas.
UVA Center for Diabetes Technology director Dr Boris Kovatchev said: “It is clear from these results, which are consistent with real-life data from thousands of current Control-IQ technology users, that this technology should be strongly considered as an option for anyone living with type 1 diabetes.”
The new diabetes-management solution has the potential to automatically monitor and control blood glucose.
The device is integrated with an insulin pump that adjusts the insulin dose automatically when needed. The pump is programmed with advanced control algorithms based on a model that uses the person’s glucose-monitoring information.
The system was approved by the US Food and Drug Administration for people aged six and older with type 1 diabetes.





