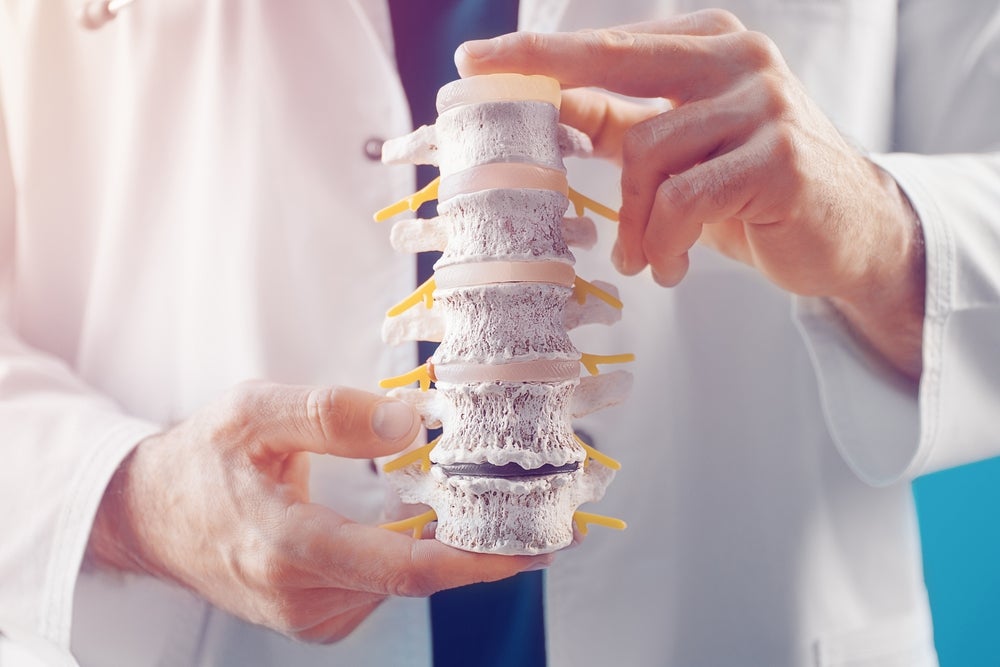
Aurora Spine has commenced its trial investigating its device for spinal fusion procedures after it enrolled its first patient for the study.
The trial (NCT05883436) will see 80 patients scheduled for anterior cervical discectomy and fusion implanted with the company’s DEXA-C device. The cervical interbody system is a porous 3D-printed interverbal l fusion device with a range of lattice density options. Data from the trial will be obtained from 40 single-level and 40 multiple-level patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The prospective, multi-centre trial will assess fusion via X-Ray at 3 months, 6 months, and 12 months post-surgery. Patient-reported outcomes, such as neck disability index and visual analogue scale for pain, will also be collected at follow-up visits.
The trial is currently recruiting at a site in Illinois, with further enrolment planned in California. Aurora Spine received multi-site selection approval from the Institutional Review Board in April 2023. Dr Sebastian Koga, principal investigator, hopes the multi-site model will provide evidence to help the device’s adoption by a wide range of clinicians for patients.
Spinal fusion procedures are usually required in patients with degenerative disc disease (DDD). Although considered a normal part of ageing, DDD can severely affect daily activities by impairing motion. In a market model by GlobalData, the interbody spinal fusion devices market is forecasted to reach $5.5bn by 2033.
The device’s lattice options mean its density can be matched to patient’s bone density. Its hollow core promotes bone integration and fusion between endplates.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The launch of the study is exciting news for Aurora and its DEXA platform technology. With this multicenter study now underway, we will be able to collect and provide important data to the medical industry about the DEXA-C device and technology platform,” said Aurora’s president, CEO and co-founder Trent Northcutt.





