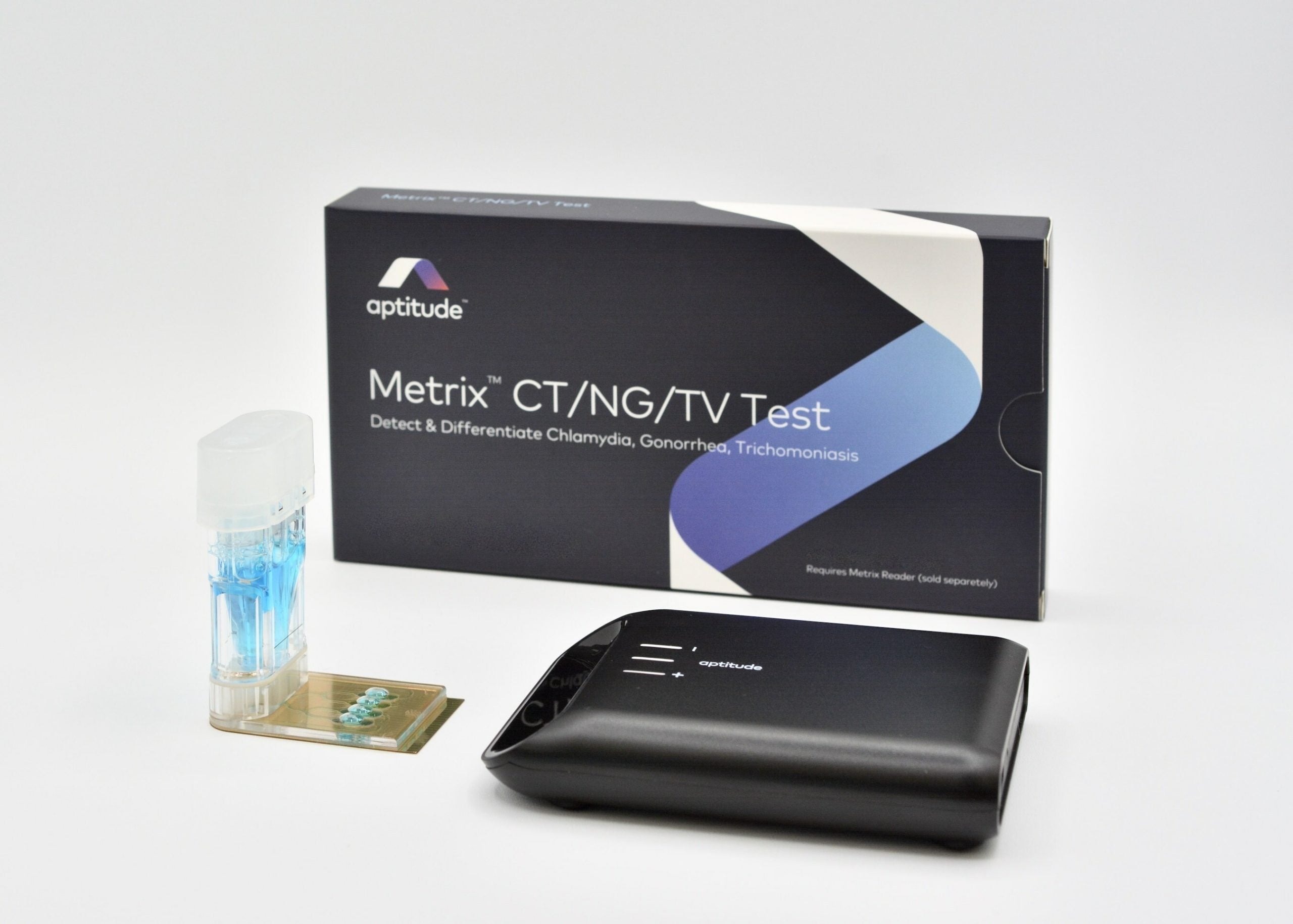
Aptitude Medical Systems is swapping Covid-19 for a different type of pathogen after the company revealed a partnership with the Bill and Melinda Gates Foundation (BMGF) to accelerate the development of its point-of-care STI diagnostic test.
The undisclosed amount of funding will help develop the new test using technology from the company’s Covid-19 testing platform. According to Aptitude, the test will be able to detect Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the UK, STI rates have been increasing at an alarming rate. In 2022, there was a 23.8% increase in new STI diagnoses in England compared to 2021. There have been drives to issue free home testing kits via post, and vending machines that dispense STI tests are being installed across universities to remove stigmatisation.
A GlobalData market model indicates that the chlamydia testing market alone could be worth $848m by 2033.
California-based Aptitude’s platform is Metrix which has been used for an over-the-counter molecular test for Covid-19. According to the company, it was the first US Food and Drug Administration (FDA)-authorised molecular diagnostic test that works with saliva or swab samples that does not require supervision by a healthcare professional.
Scott Ferguson, CEO of Aptitude Medical Systems, said: “This generous funding from the Bill & Melinda Gates Foundation endorses the promising capabilities of our Metrix platform and will achieve a truly meaningful public health impact. The platform’s proven over-the-counter, user-friendly interface paves the way for a new generation of diagnostics that solves access to critical health information – not only in the U.S. but especially globally where transforming health equity demands a disruptive solution.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn April 2023, Aptitude received a $53.7m grant from the Biomedical Advanced Research and Development Authority (BARDA) to continue building additional tests for infectious diseases.



