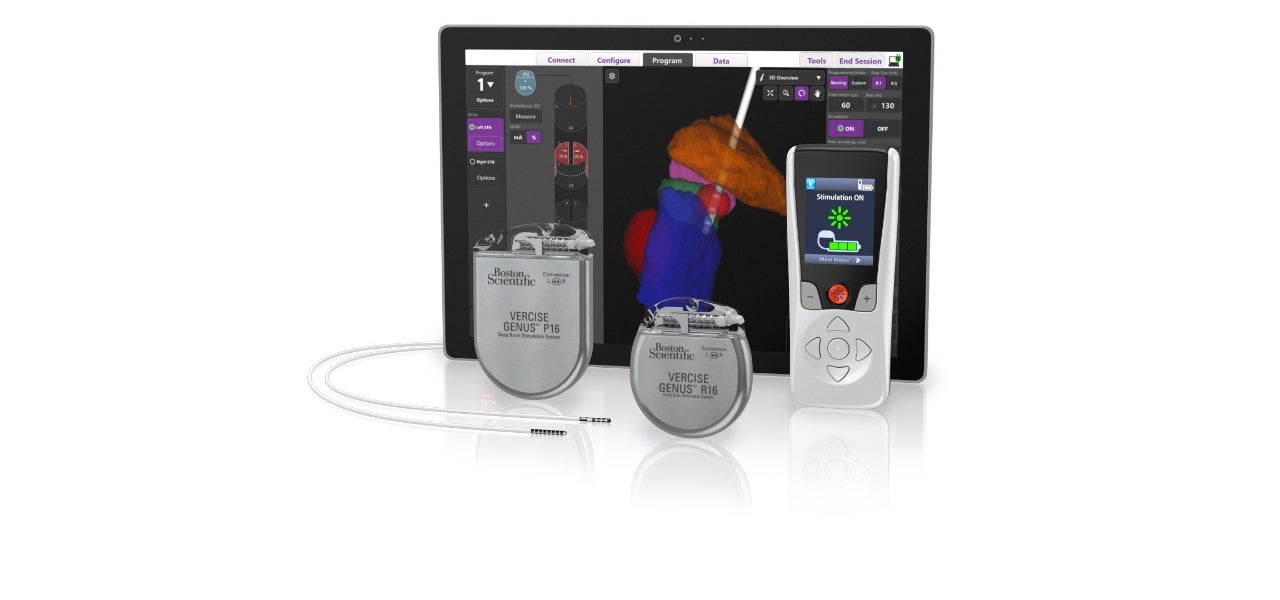
Boston Scientific has initiated a limited market release of the fourth generation Vercise Genus Deep Brain Stimulation (DBS) system in Europe, following the receipt of CE Mark approval for the system.
Vercise Genus system is designed for the treatment of symptoms of Parkinson’s disease (PD), essential tremor and dystonia. The system delivers precisely targeted electrical stimulation in the brain to offer optimal symptom relief.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The new Vercise Genus system incorporates clinician software that optimises programming with integrated visualisation. The software uses patient imaging through the company’s exclusive partnership with Brainlab.
Additionally, it comes with added features for patients, including a low-profile two-in-one extension with the option of abdominal placement.
Boston Scientific Neuromodulation senior vice-president and president Maulik Nanavaty said: “For patients, the Vercise Genus DBS System continues the tradition of small, thin devices and it provides Bluetooth programming, which is important during times of social distancing.
“Combined with the option of a 25-year rechargeable battery, as well as the expanded MRI conditional feature available on our primary cell devices, patients can find the best option to suit their specific needs.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn Europe, Vercise Genus DBS system is approved for use in unilateral or bilateral stimulation of the subthalamic nucleus or internal globus pallidus for treatment of levodopa-responsive PD and for treatment of intractable primary and secondary Dystonia for people aged seven years and older.
Thalamic stimulation, using the Vercise Genus system, is indicated for the suppression of tremor not adequately controlled by medications in patients diagnosed with essential tremor or PD.
Over ten million people are living with PD worldwide. This progressive and neurodegenerative disorder leads to stiffness, slowness and tremors due to the decrease of dopamine in the brain.
Dystonia, which affects over half a million men, women and children across Europe, is considered to be the third most common movement disorder after essential tremor and PD.





