Boston Scientific has announced financial results for the full year 2023, with reported GAAP net income attributable to the company’s common stockholders reaching $1.57bn, an increase from $642m in the previous year.
The company’s net sales surged to $14.24bn, marking a 12.3% growth on a reported basis and 13.1% on an operational basis, compared to the prior year.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Diluted earnings per share (EPS) for FY2023 stood at $1.07, up from $0.45 in the 2022 financial year (FY) while full-year adjusted EPS rose to $2.05 from $1.71 in 2021.
Boston Scientific chairman and CEO Mike Mahoney said: “I am grateful to our global team and proud of our exceptional results in 2023.
“We are excited about our future and long-range plans as we deliver on our mission to transform patient lives.”
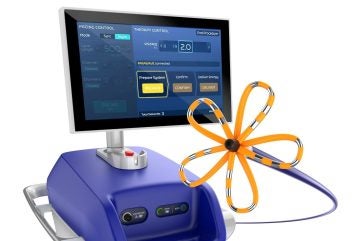
In another development, the FDA approved the company’s FARAPULSE PFA system.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis system is a new treatment option for people with drug-refractory, recurrent, symptomatic, paroxysmal atrial fibrillation (AF). It is a substitute for traditional thermal ablation treatments.
It operates using non-thermal electric fields to selectively ablate heart tissue while minimising damage to adjacent structures.
The system includes the FARAWAVE Ablation Catheter, the FARASTAR Ablation Generator, and the FARADRIVE Steerable Sheath, which is used in conjunction with the VersaCross Connect Access Solution.
Clinical evidence from the ADVENT trial supported the system’s efficacy and safety comparing the FARAPULSE PFA System directly with standard-of-care ablation.
The 12-month data showed that the device’s performance was on par with conventional thermal ablation.
Boston Scientific Electrophysiology president Nick Spadea-Anello said: “The approval of the FARAPULSE PFA system marks an important milestone for the millions of people living with paroxysmal AF and is an incredible opportunity to bring the first PFA system designed and built solely for this type of ablation therapy to physicians in the US.”


