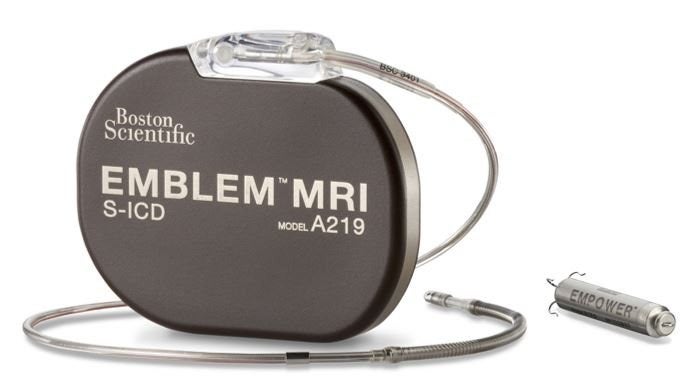
Boston Scientific has started a trial to assess the safety and effectiveness of the mCRM Modular Therapy System.
The mCRM System comprises two cardiac rhythm management (CRM) devices: the EMBLEM MRI Subcutaneous Implantable Defibrillator (S-ICD) System and the EMPOWER Modular Pacing System (MPS).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The proven S-ICD System is designed to prevent sudden cardiac death among patients.
Meanwhile, the EMPOWER MPS includes a leadless pacemaker and delivery catheter to complement the S-ICD System by adding a modular option for patients who develop anti-tachycardia pacing (ATP) requirements.
The two devices are designed to work together to coordinate therapy.
The study, named MODULAR ATP, seeks to assess the communication between the two devices in a bid to expand treatment options for patients requiring an Implantable Cardioverter Defibrillator (ICD).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPreclinical studies indicate that the mCRM System provides excellent performance at up to 18 months.
MODULAR ATP will enrol up to 300 patients at around 50 centres in the US, Canada and Europe. The patients will either be guideline-indicated for an ICD or have an EMBLEM or EMBLEM MRI S-ICD System already implanted.
The objectives of the trial include assessment of the system- and procedure-related complication-free rate of the EMPOWER MPS, the communication between the two systems and the pacing capture thresholds of the EMPOWER MPS.
Boston Scientific rhythm management and global health policy chief medical officer Kenneth Stein said: “Since the EMPOWER MPS device can be delivered percutaneously via a minimally invasive approach without the use of leads, the mCRM System could preserve many of the benefits of the S-ICD System while offering an option for patients who subsequently develop a pacing requirement.
“The components of the system are designed to work in concert with each other, regardless of when implanted, giving physicians the ability to provide personalised patient care today while keeping options open in the future.”
Boston Scientific is a Massachusetts-based medical device company. In October, it signed an agreement to acquire Baylis Medical Company and its cardiology business for an upfront payment of $1.75bn.





