CardioRenal Systems has secured Breakthrough Device Designation from the US Food and Drug Administration (FDA) for its RenalGuard Therapy device.
RenalGuard Therapy is indicated for preventing acute kidney injury (AKI) in people who are at risk of developing Cardiac Surgery Associated AKI (CSA-AKI).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has been designed to protect the kidneys through personalised, active hydration.
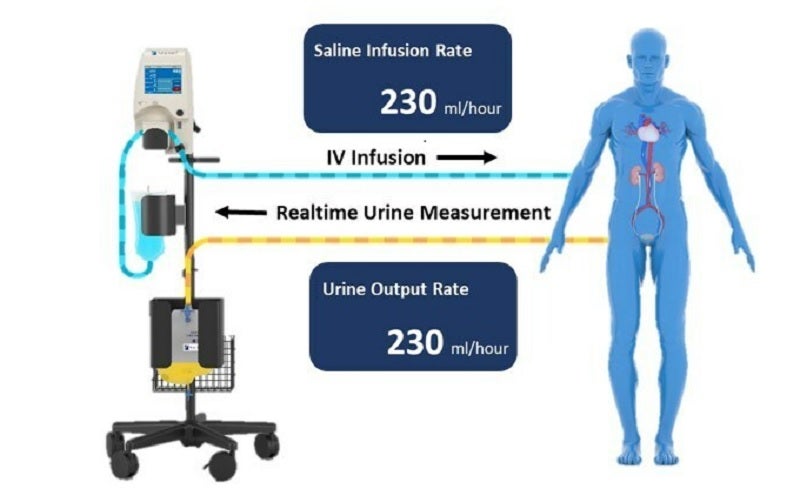
The device maximises urine output while balancing hydration with real-time urine output monitoring and IV infusion using a smart re-hydration system.
CardioRenal Systems CEO Ilya Budik said: “We are thrilled to receive the Breakthrough Device Designation and appreciate all the hard work that our team put in to get us here.
“We are looking forward to working closely with the FDA and our partners to facilitate the initiation of the upcoming US pivotal study.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The high prevalence of AKI in cardiac surgery today is a well-known risk. We look forward to building further clinical validation that RenalGuard Therapy can provide the solution to reduce the prevalence of CSA-AKI, the length and cost of hospitalisation, and most importantly to improve patients’ quality of life.”
Compared to the standard of care, RenalGuard was found to significantly reduce the incidence of Contrast-Associated AKI in two investigator-sponsored studies.
The system comprises a console and RenalGuard Single-Use Set for infusion and urine collection.
The single-use set includes a urine collection set and infusion set, which connect to a patient’s Foley catheter and standard IV catheter, respectively.
The console measures the urine volume in the collection set and delivers an equal volume of hydration fluid through the infusion set.
It works based on patented software and electronic weight measurements for monitoring the urine volume and controlling the fluid infusion rate.





