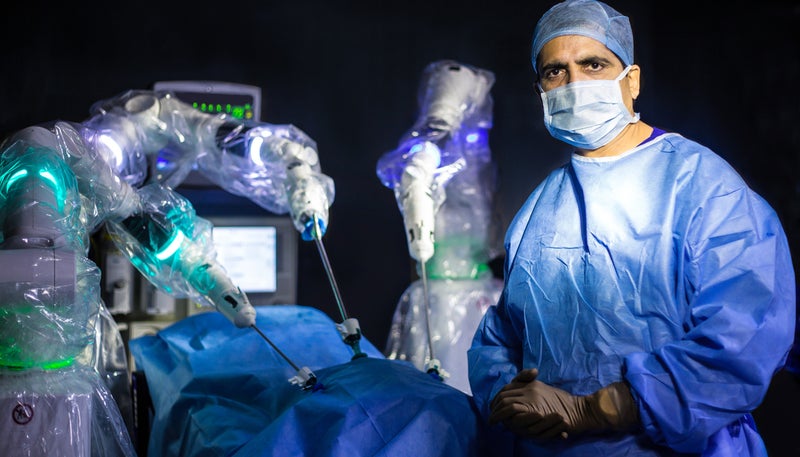
UK-based medical equipment maker CMR Surgical has announced the completion of the first series of human surgical procedures using its Versius surgical robotic system as part of a clinical study in India.
Conducted at Mangeshkar Hospital & Research Center in Pune, the procedures included 30 laparoscopies across minor, intermediate and major gynaecological and upper gastrointestinal (GI) indications.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The surgeons did not find any Versius-related adverse events following a 30-day follow-up.
CMR Surgical chief medical officer Mark Slack said: “This first-in-human series is a significant milestone in bringing Versius to operating theatres around the world. These initial results are positive and we look forward to further advancing our mission to bring the benefits of minimal access surgery to everyone who needs it. This series is part of our drive for the responsible introduction of surgical robotic systems that puts safety and effectiveness above all else.”
Versius is a next-generation surgical robotic system designed to address the complexities and offer improved access during minimal access surgeries.
It features a small form and individually cart-mounted arms, which enable transportability between operating rooms and hospitals/clinics.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe single-centre, prospective cohort study is intended to assess the safety and performance of the system in robotically-assisted surgery across various abdominal and pelvic surgeries.
Primary endpoint of the study is the rate of unplanned conversion of procedures to other minimal access surgery or open surgery.
In addition, the study will monitor secondary outcomes such as operative time, estimated blood loss and blood transfusion rate, intra-operative complications and length of hospital stay.
The study will also collect 90-day mortality statistics.
Findings will be included in the CMR Surgical Registry, a data registry intended to gather clinical performance and safety data on all Versius surgical procedures performed in all geographical locations.
After completing the first-in-human series, the company plans to initiate clinical introduction of the Versius system for use in minimal access surgery globally.





