US-based medical device company Emboline completed enrolment of patients in SafePass 2 clinical study of Emboliner Embolic Protection Device.
The device provides embolic protection of the brain and body during transcatheter aortic valve replacement (TAVR).
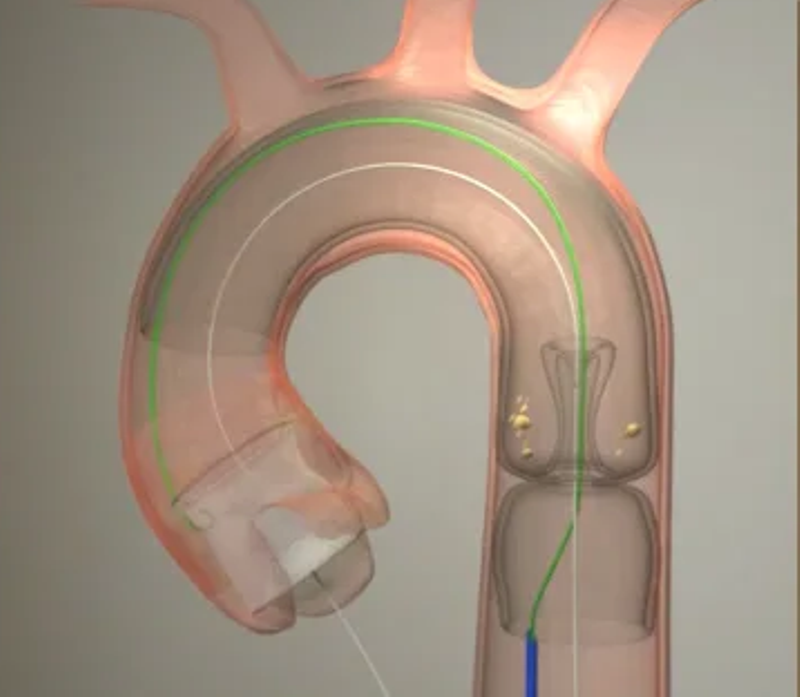
It is a cylindrical Nitinol mesh device that covers the complete arterial bed of all three cerebral branches and non-cerebral vessels.
Furthermore, it includes an expandable access port whereby TAVR devices are passed, allowing the machine to capture and contain debris during the whole TAVR procedure.
SafePass 2 is a non-randomised, multicentre, open-label study carried out at three centres in New Zealand.
The study is intended to examine the safety and technical performance of the second-generation Emboliner device.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataEarly results from the first 24 patients showed outstanding safety profile and technical performance, along with significant debris capture and removal in all patients, according to the company.
The study assessed the quantity of debris collected from patients, finding an average of 250 particles ≥150um in size removed from each individual. Over half of had debris ≥1mm in size, while one in four cases had debris ≥2mm in size.
Emboline states that final data analysis is currently underway for the full study population, with data used later this year to file for CE Mark.
Emboline CEO Scott Russell said: “The amount of debris captured and removed from TAVR patients using this device is several times greater than any first-generation cerebral protection device has shown to date.
“Results from this study demonstrate the potential for Emboliner to significantly improve upon first-generation cerebral protection to protect both the brain and body, which is increasingly important as TAVR moves to younger and healthier patients.
“With these data, we will begin the processes of securing CE Mark and seeking approval to begin our pivotal US clinical study.”







