CorNeat Vision has obtained 510(k) clearance from the US Food & Drug Administration (FDA) for its synthetic tissue substitute, EverPatch.
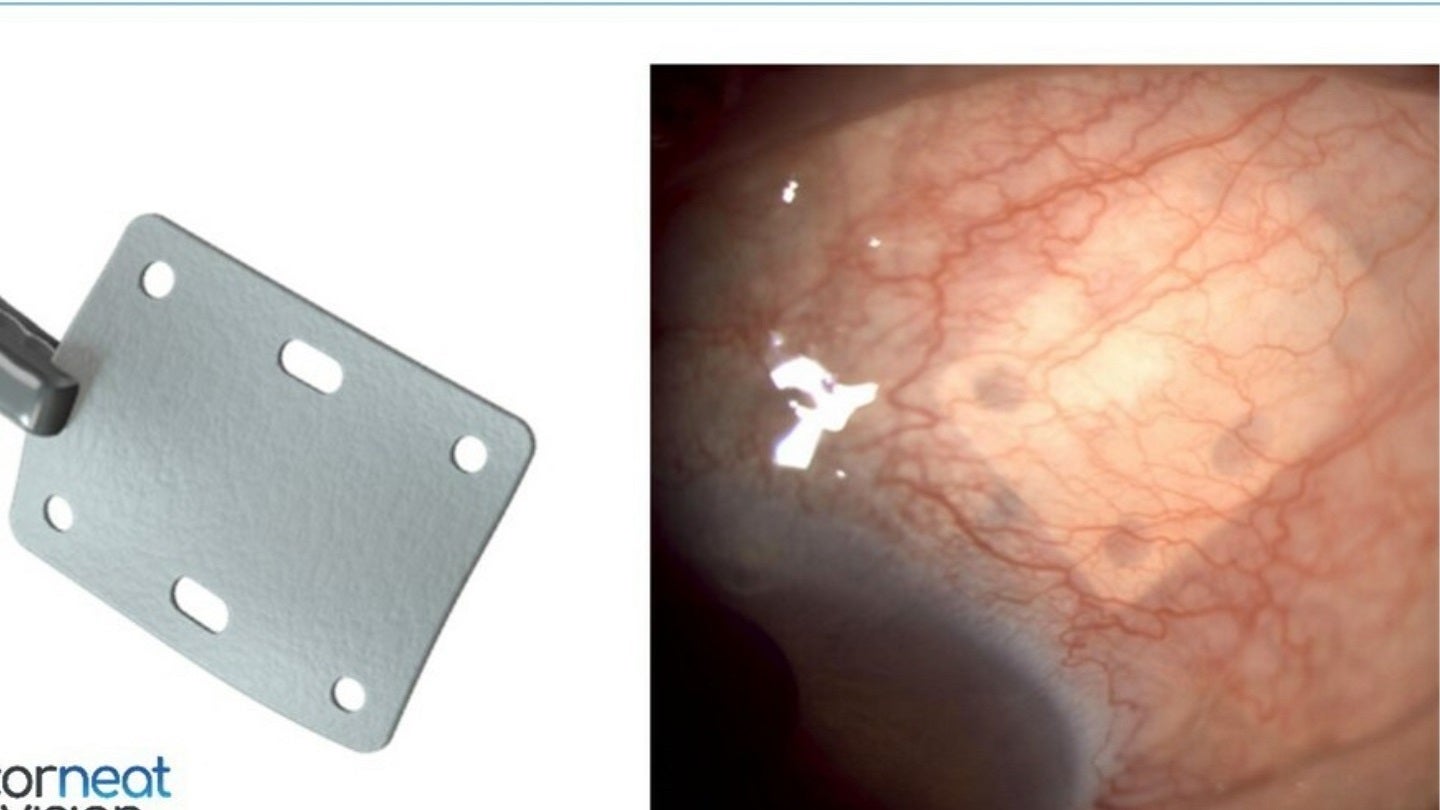
CorNeat EverPatch is claimed to be the world’s first synthetic and non-degradable tissue-integrating matrix designed for application in ophthalmic surgeries.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It consists of a non-woven, polymer matrix that combines with surrounding tissue.
CorNeat EverPatch helps strengthen the sclera and assists in the physical restoration of the ocular surface.
CorNeat Vision co-founder and chief medical officer Dr Gilad Litvin said: “The ideal graft material should be long-lasting, sterile, immunologically inactive, cosmetically acceptable and readily available.
“The CorNeat EverPatch was designed with these goals in mind. Our novel ophthalmic patch is significantly thinner than processed patch tissue and provides better handling as it does not ‘cheese wire’ when sutured and has holes that allow for accurate positioning and anchoring.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataDesigned to meet the critical requirements of ocular surgeons, the synthetic tissue substitute offers a sterile and non-degradable solution for patients across the world.
The CorNeat EverPatch is set to supersede the use of donor and processed tissue in ocular surgeries, which often carries the risk of disease transmission.
The company plans to initially roll out this synthetic tissue substitute in major ophthalmic centres in the US from the third quarter of this year.
CorNeat Vision CEO and research and development vice-president Almog Aley-Raz said: “EverMatrix presents a significant business opportunity as it is the only synthetic non-degradable patch material in ocular surgery.
“This biocompatible material has the potential for wider use in soft tissue reinforcement, biomechanical integration of implants with surrounding tissue, fabrication of membranes and concealment of implants and sensors.”





