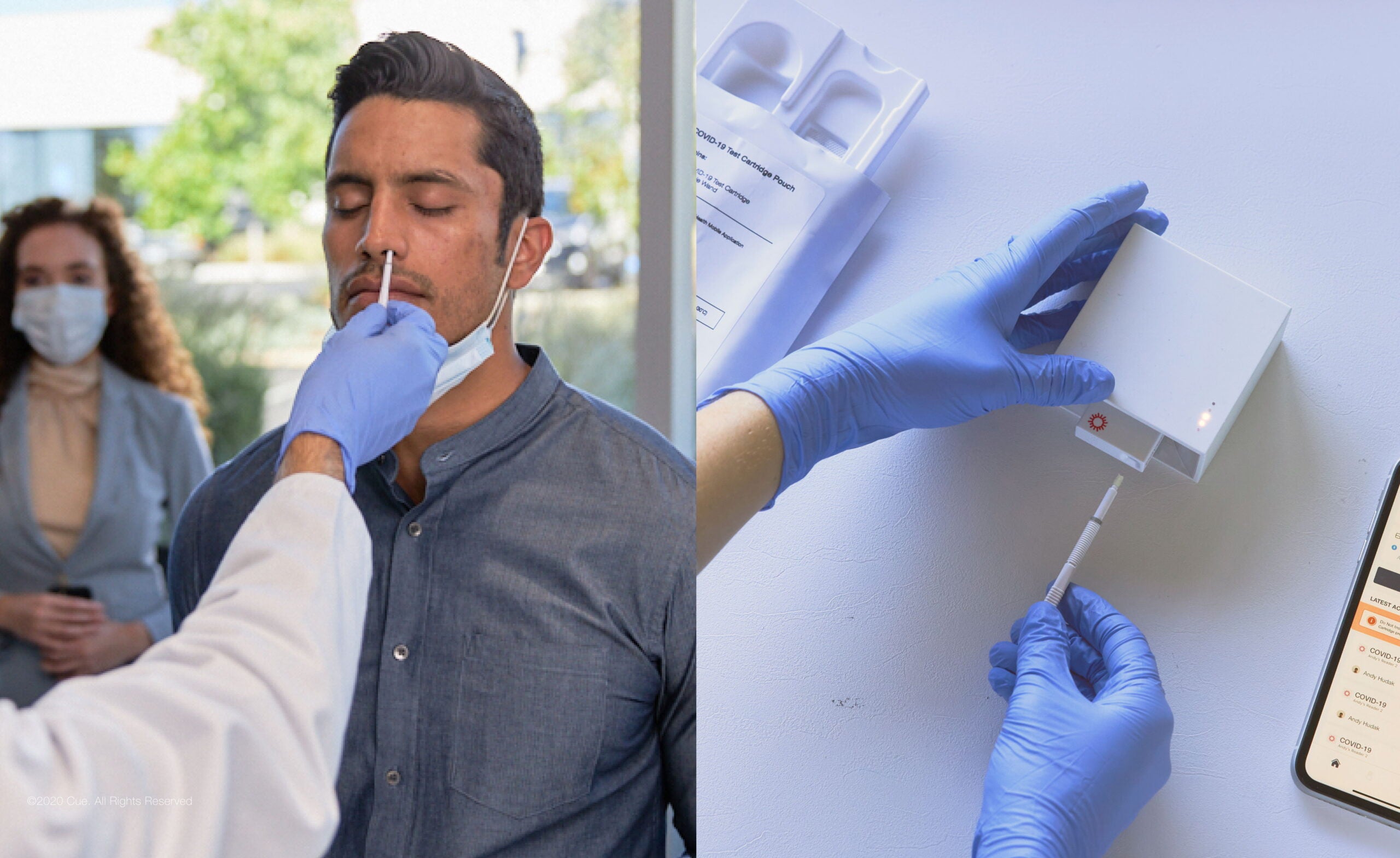
Healthcare technology company Cue Health has received the CE mark for its molecular point-of-care Covid-19 test for sale and distribution in the EU.
The Cue COVID-19 Test is a highly sensitive and specific nucleic acid amplification test (NAAT) that works on the Cue Health Monitoring System.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It delivers results on a connected mobile smart device with the help of the Cue Health App in 20 minutes.
The test comprises a single-use, self-contained molecular assay, Cue Test Cartridge, and a lower nasal swab, Cue Sample Wand, for sample collection in a minimally invasive manner.
The reusable, battery-operated Cue Cartridge Reader runs the Cue Test Cartridge and delivers results to the app.
The app is an interface for providing test information and instructions and displaying the test results.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCue Health chief product officer Clint Sever said: “The Cue COVID-19 Test brings lab-quality performance to the point-of-care, enabling fast clinical decisions for patient triage and care.
“Accurate and rapid diagnosis is critical for infection control as businesses and communities aim to reopen and remain open by practising safety protocols and regular testing, even with the rollout of the vaccine underway.”
A portable and connected diagnostic platform, the Cue COVID-19 Test and Cue Health Monitoring System fit in the palm and offers rapid and precise lab-quality molecular testing at point-of-care.
Currently, Cue’s Covid-19 tests are used in point-of-care settings in the US, for example, in K-12 schools, essential businesses, nursing homes, and other congregate-care facilities, hospitals, physicians’ offices, and dental clinics.
Last June, Cue Health received emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for its portable molecular Covid-19 test two days after announcing the closure of a $100m Series C round for its development.





