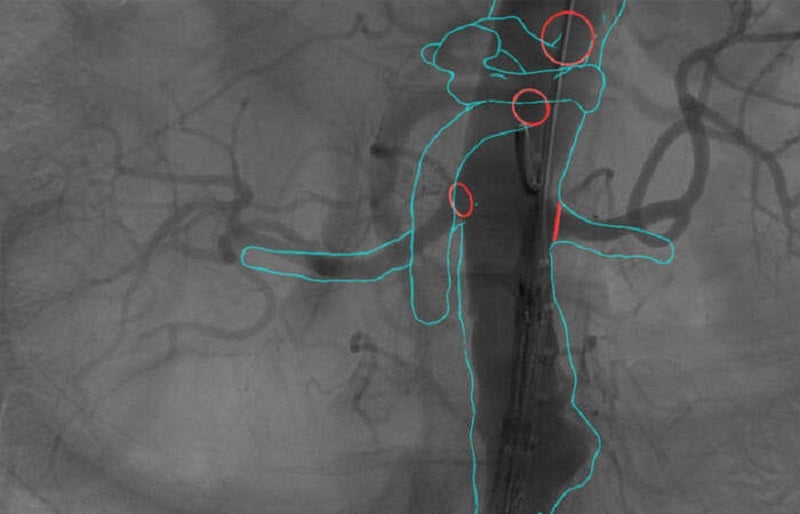
Cydar Medical and King’s College London have enrolled the first participant in the ARIA study of artificial intelligent (AI) image fusion system, Cydar EV Maps.
Cydar EV Maps has been developed to guide aortic endovascular aneurysm repair (EVAR).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to the company, EVAR is preferred to the alternative of open aortic surgery due to shorter hospital stays, fewer complications after surgery and advantages in patient survival.
The patented computer vision helps to plan and guide EVAR by automatically overlaying the map on live X-ray images.
Real-time imaging is used to update the 3D Map throughout procedures, particularly when instruments and guidewires deform blood vessels.
Cydar EV Maps is said to be the first product from the company’s integrated software solutions system.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt integrates with existing imaging hardware that is present in hospitals where EVAR surgery is performed.
King’s College London clinical senior lecturer and ARIA study principal investigator Dr Rachel Clough said: “Our central hypothesis is that digital technology, specifically Cloud-computing and AI, can be used to assess and learn from large volumes of data to inform clinical decision making and has the potential to improve the predictability of individual patient outcomes and the consistency of outcomes in the NHS.”
The randomised controlled trial has been designed to evaluate the technical, clinical and cost effectiveness of Cydar EV Maps compared to the standard guides for aortic EVAR.
The first participant was enrolled at the Liverpool University Hospitals NHS Foundation Trust.
Approximately 340 participants with a clinical diagnosis of abdominal aortic (AAA) or thoracoabdominal aortic aneurysm (TAAA) who are suitable for endovascular treatment will be enrolled at ten sites across the UK.
The trial participants will be followed for one year.





