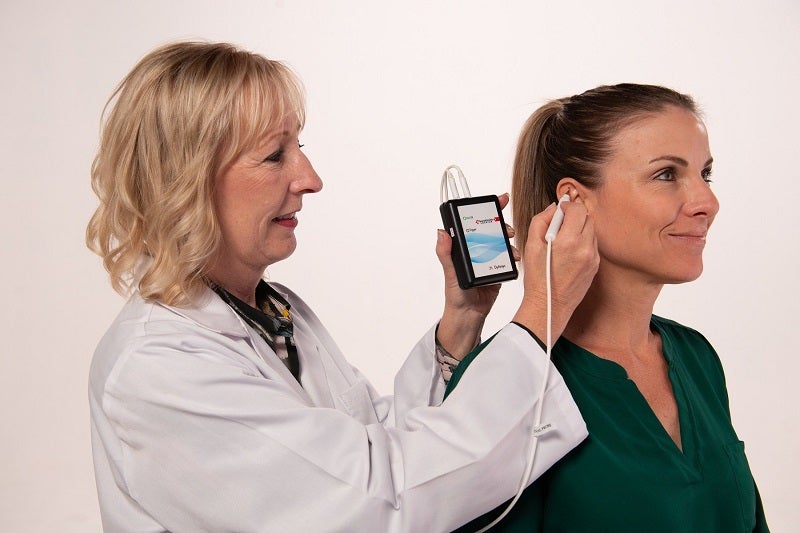
DyAnsys has received approval from the US Food and Drug Administration (FDA) for its percutaneous electrical nerve stimulator (PENS) system, Primary Relief, to treat post-cardiac surgery pain.
Primary Relief has been designed for symptomatic relief of pain for up to three days after cardiac surgery using auricular neurostimulation treatment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Placed in the ear, the wearable, battery-operated PENS device administers periodic low-level electrical pulses to the ear for more than 72 hours.
Through a wire assembly and stimulation needles, the electrical pulses are delivered to the branches of the cranial nerves that are located on the ear.
DyAnsys CEO Srini Nageshwar said: “This ground-breaking device allows for significant pain relief without the use of narcotics.
“By reducing or avoiding the use of opioids after surgery, the risk of addiction is reduced. We look forward to connecting with physicians and patients to make this option available after cardiac surgery among other applications.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company stated that Primary Relief’s effectiveness in treating post-cardiac surgery pain was shown in a double arm, single-centre, prospective, randomised study, which was conducted with 60 patients.
The findings from the study demonstrated that minimally invasive nerve stimulation intervention using the DyAnsys neurostimulation device reduced pain compared to a placebo device.
Primary Relief also reduced the need for analgesics after surgery.
Its nonclinical testing includes performance bench testing, biocompatibility testing, software verification and validation, and electrical safety (electromagnetic compatibility and safety).
In July, the neurostimulation device received FDA approval to treat pain after Cesarean section (C-section) delivery.
DyAnsys also provides two other PENS devices, First Relief and Drug Relief.
First Relief has received FDA approval to treat diabetic neuropathic pain, and Drug Relief is approved as an aid for drug withdrawal.





