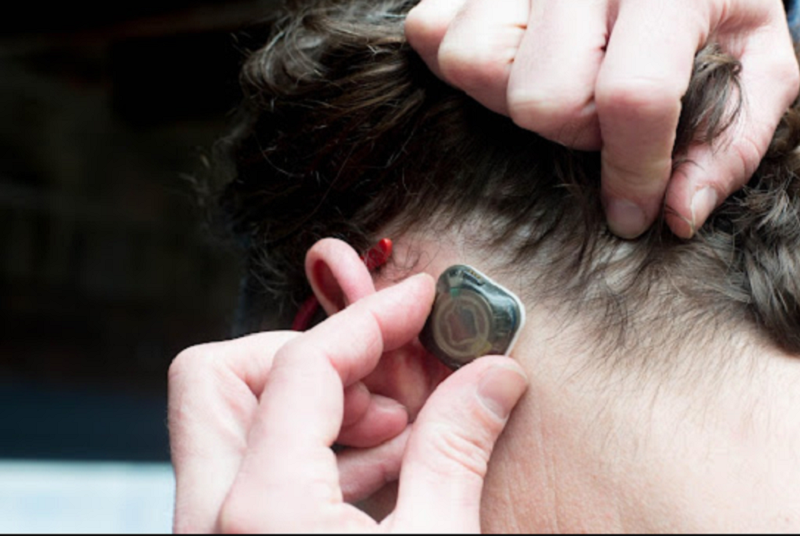
Digital health company Epitel has raised $12.5m in Series A funding for the development and commercialisation of REMI, its wearable, wireless electroencephalography (EEG) monitoring system for detecting seizures.
The funding round was co-led by Genoa Ventures and Catalyst Health Ventures (CHV).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Additionally, OSF Ventures, Dexcom, MedMountain Ventures, Salt Lake City Angels and Wavemaker 360 also participated.
Previously, Epitel secured more than $7.5m, which was funded by the National Institutes of Health (NIH) and Epilepsy Foundation.
The company initially received US Food and Drugs Administration (FDA) approval for the REMI wearable EEG brain wave monitor sensor and remote access software for the detection of seizures last year.
The Epitel’s REMI approval is for use in critical care units and hospital emergency rooms across the US.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt can easily be used by nurses and hospital technicians.
The EEG data collected can be connected immediately to a Cloud-based software platform that is available to neurologists for monitoring and reviewing seizures at any time and from any location.
Epitel CEO Mark Lehmkuhle said: “Epitel’s first FDA-cleared product, REMI, has the potential to revolutionise the diagnosis, treatment and management of seizures within the hospital. With Epitel, patients, no matter their geography, may have access to essential EEGs during the most critical times of need.
“We intend to further expand our product pipeline for use outside the hospital by people living with epilepsy and other seizure conditions. We are honoured to have the support of Catalyst Health Ventures, Genoa Ventures and a strong investment syndicate in our first financing.”
During the hospital journey, the EEG system can be used to monitor patients continuously for 48 hours.





