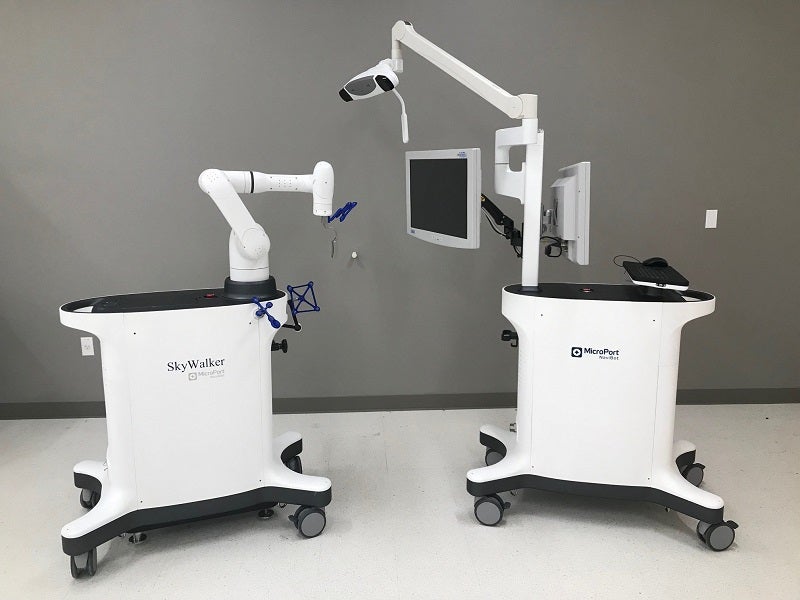
The US Food and Drug Administration (FDA) has granted 510(k) clearance for MicroPort Navibot’s robot-assisted SkyWalker System for orthopaedic applications.
The new platform is said to be compatible with the Evolution Medial-Pivot Total Knee System and will initially be used in total knee replacements.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The SkyWalker System helps surgeons formulate a personalised patient implant plan from preoperative CT scan anatomical data and specific implant data before the actual surgery.
It has the technical advantages of providing accurate operation and efficient coordination.
The new robot-assisted platform enables precise implant positioning that depends on actual patient alignment and anatomy during the surgery
It then allows the surgeon to proceed to resectioning using a lightweight mechanical arm, resulting in improved accuracy, precision and efficiency.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe device can provide information that helps achieve the desired joint line reconstruction and also delivers data to optimally balance soft tissues.
In the future, MicroPort Navibot plans to develop other orthopaedic applications with MicroPort Orthopedics to offer more comprehensive orthopaedic solutions.
The company has constructed a full technology research facility, which is designed based on clinical needs.
With medical industry design capabilities, the facility is said to benefit from core technological advantages and a high-dexterity, lightweight, self-developed mechanical arm combined with intelligent planning and navigation algorithms.
In January, MicroPort Scientific’s subsidiary MicroPort CRM received Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) approval for Alizea, the company’s latest range of pacemakers.
The implantable pacemakers are paired with the company’s SmartView Connect home monitor.





