The US Food and Drug Administration (FDA) has granted an Emergency Use Authorization (EUA) to Becton, Dickinson and Company’s (BD) Covid-19 rapid, lateral flow antigen test for use in at-home settings.
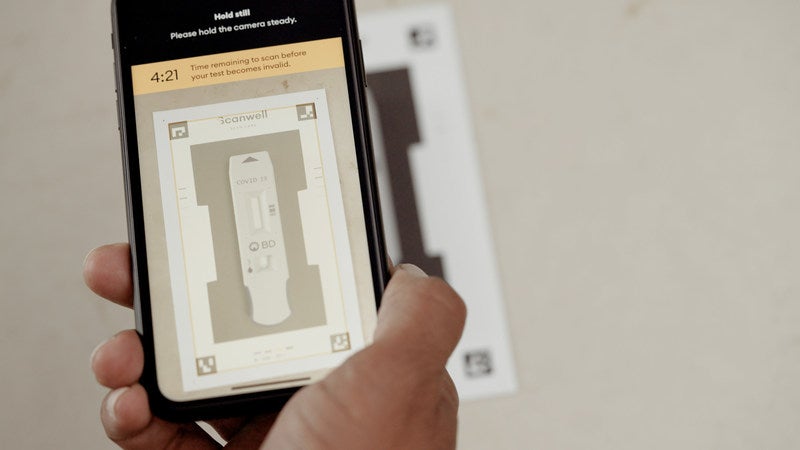
The BD Veritor At-Home COVID-19 Test leverages computer vision technology in a smartphone to read and deliver a digital display of test results.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It uses a pain-free nasal swab and simple mobile application from Scanwell Health to provide consistent results in 15 minutes.
Available on iOS as well as Android, the app gives stepwise directions on the nasal swab sample collection and its transfer to the test stick.
On transferring the sample, the smartphone’s camera is used to capture, assess and read the results, eradicating the human subjectivity of a test that is read visually.
BD noted that the self-test is intended for use by individuals aged 14 years or above and does not require a prescription or time-consuming lab processing.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFurthermore, the test can be used in children as young as two years if an adult obtains the samples.
In the initial stage, the digitally read test will be provided for businesses, schools and governments that require a self-testing option for employees or students.
BD Life Sciences president Dave Hickey said: “The rise in Covid-19 cases from the Delta variant has increased the demand for at-home testing and the BD Veritor At-Home COVID-19 Test is an easy-to-use test with definitive digital results that is ideal for use in the home.
“New mandates from governments and businesses are specifying the need for periodic testing for those who cannot or chose not to be vaccinated, and this new test may help businesses, governments or schools fulfil those requirements.”
Meanwhile, BD has introduced a fully automated high-throughput molecular diagnostic system that merges robotics and sample management software algorithms.
The BD COR PX/GX System can automate the whole infectious disease molecular laboratory workflow, including sample processing and diagnostic test results, for core as well as other centralised laboratories in the US.
In March, the FDA granted EUA to BD’s new rapid antigen test that can identify SARS-CoV-2, influenza A and influenza B in a single test.





