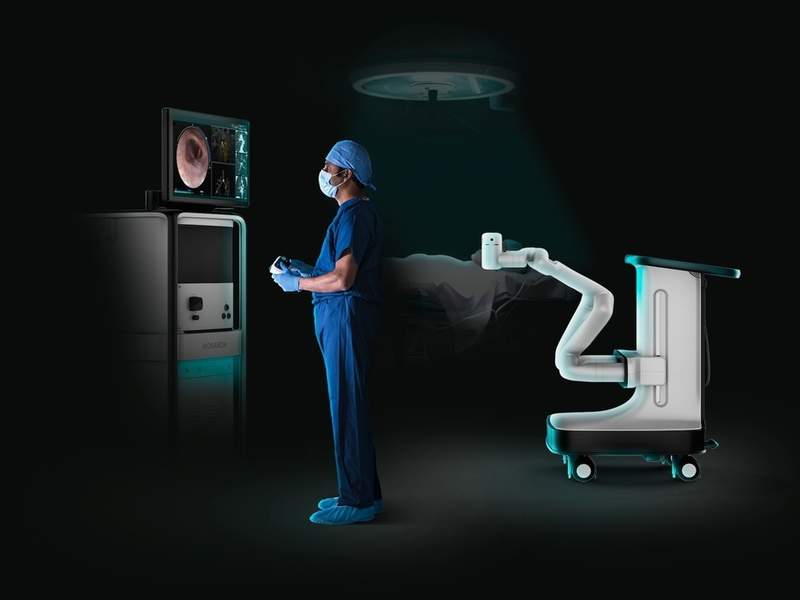
The US Food and Drug Administration (FDA) has cleared medical equipment developer Auris Health’s Monarch Platform, which has been designed to enhance bronchoscopy.
The new solution combines robotics with software, data science and endoscope advancements to increase patient outcomes and physician capabilities while lowering healthcare costs.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Meant for diagnostic and therapeutic bronchoscopic procedures, the platform initially aims to deliver precise diagnosis and treatment of even small and hard-to-reach lung nodules in cancer patients.
As the majority of lung cancer cases could not be diagnosed until advanced stages, 90% of people affected with this disease fail to survive.
While many diagnostic options are available to detect the cancer, they are said to lack in terms of accuracy, safety or invasiveness.
The new platform features a controller-like interface that is used by physicians to navigate flexible robotic endoscope with better reach, vision and control to the periphery of the lung.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt combines standard endoscopic pulmonary views with computer-assisted navigation built on 3D models of the patient’s lung anatomy to offer uninterrupted bronchoscopic vision till the end of the procedure.
Auris Health CEO Dr Frederic Moll said: “The Monarch Platform is designed to address the limitations of current technology with the introduction of a new era of flexible robotics.
“With this FDA clearance, we intend to deliver on the promise of improving patient care, starting with earlier and more accurate diagnosis of pulmonary nodules.
“We envision additional uses for the technology across future endoscopic clinical indications.”
The company focuses on developing solutions to improve physician capabilities, minimally invasive techniques and patient outcomes. It combines robotics, micro-instrumentation, endoscope design, sensing and data science into a single platform for enhanced medical intervention.
So far Auris Health has raised more than $500m in equity capital to support its work.





