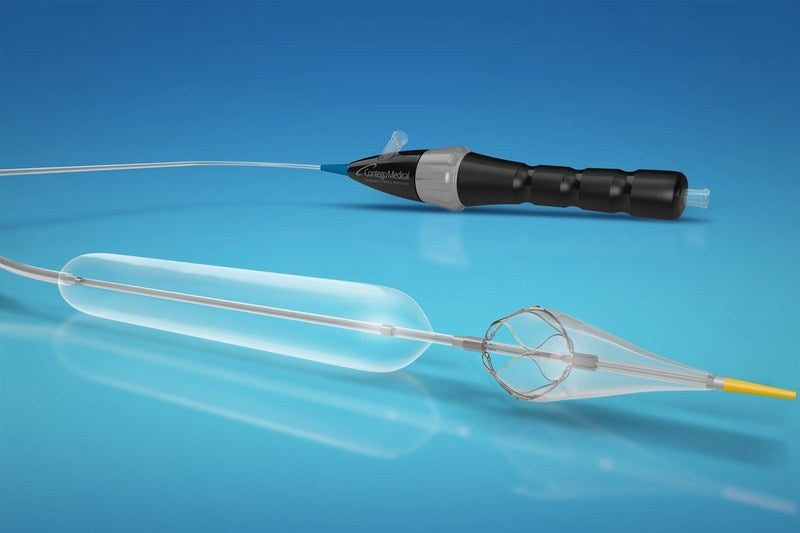
Medical devices firm Contego Medical has received the US Food and Drug Administration (FDA) 510(k) clearance for the Vanguard IEP Peripheral Balloon Angioplasty System, which treats peripheral vascular disease.
Vanguard has been designed for percutaneous transluminal angioplasty (PTA) as well as the capture and removal of embolic material during angioplasty for the femoral, iliac, popliteal and profunda arteries.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Contego said that the system protects the lower limbs during angioplasty procedure and avoids the need for other devices or exchanges.
The device features Integrated Embolic Protection (IEP) technology with a peripheral angioplasty balloon and distal embolic filter to enable simultaneous treatment and capture and retrieval of any dislodged embolic particles.
Along with an over-the-wire design having a sheathless integrated 150µ pore filter distal to the angioplasty balloon, the device comes with in-vivo adjustability for different vessel sizes.
Contego Medical founder and CEO Ravish Sachar said: “As the patient population with peripheral arterial disease continues to expand and become more complex, we believe our technology will play a critical role in protecting vulnerable patients from embolic events and thus improve procedural outcomes.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataWhen assessed in the ENTRAP 112-patient post-market registry in Europe, all the participants met primary safety and efficacy endpoints at discharge and 30 days.
ENTRAP Study principal investigator professor Thomas Zeller said: “In our experience, the device has performed exactly as intended and we are impressed with the ease of use of the system, with no more exchanges required than in a typical angioplasty procedure.”
Contego is focused on the development of medical devices for neurovascular, cardiovascular and peripheral vascular procedures.
Besides Vanguard, the company’s portfolio includes Paladin Carotid System and Corguard Coronary System based on the IEP technology.





