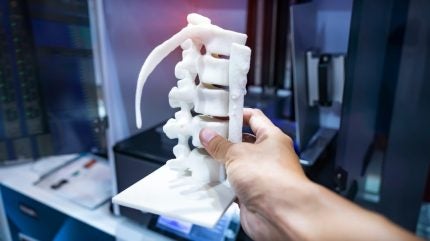
Vy Spine has received clearance for its 3D printed LumiVy OsteoVy PEKK Lumbar IBF device from the US Food and Drug Administration (FDA).
The device is indicated for intervertebral body fusion for use at either one level or two contiguous levels in the lumbar spine, from L2 to S1, as a treatment for degenerative disc disease (DDD) with up to grade 1 spondylolisthesis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This also marks the second FDA approval in Vy Spine’s OsteoFab product range. The company’s ClariVy OsteoVy PEKK Cervical IBF device received clearance from the US regulatory agency in November 2023. It is also indicated for intervertebral body fusion and as a treatment for degenerative disc disease but in the cervical spinal area, from C3 to T1.
Vy Spine notes that the device combines the osseointegration properties of the 3D-printed OXPEKK material and the novel OsteoVy lattice structure. The OXPEKK material offers advantages over the PEEK material including “bone ingrowth, no radiographic interference, no fibrotic tissue membrane formation, a significant increase in bony apposition over time, and significantly higher push-out strength”.
The Vy Spine’s device was made using 3D-printed Oxford Performance Materials’ OsteoFab technology in an OsteoVy lattice structure.
The use of 3D printing for manufacturing has been increasing in recent years. 3D printing offers various advantages, including customisation, reduced production costs, and quick turnaround, all of which are expected to propel the medical 3D printing market to generate $4bn in 2026, as per GlobalData analysis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe spinal fusion orthopaedic device market is projected to grow over $8.9bn in 2030, as per a GlobalData market model. The interbody spinal fusion device segment will grow to roughly $4.2bn in 2030. Multiple companies have made moves to capitalise on 3D printing technology to formulate spinal implants.
In August, NanoHive Medical raised $7m in Series C financing to develop and commercialise its 3D-printed titanium spinal interbody fusion devices. In May 2023, Tissium raised €50m ($53.8m) in Series D financing to develop a 3D-printed implantable device and on-demand activated adhesive for suture-less nerve repair.





