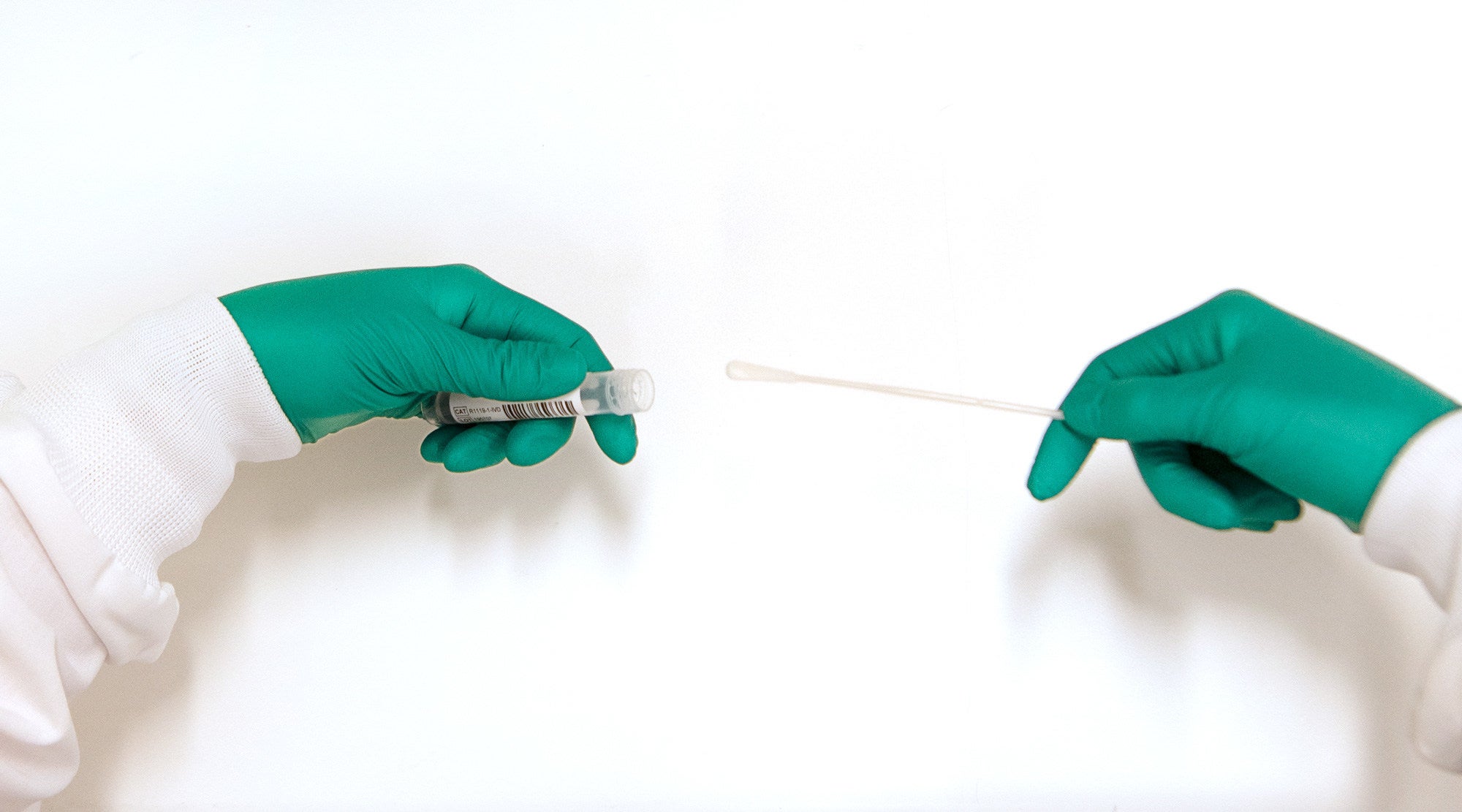
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Zymo Research’s DNA/RNA Shield Collection Tube as a Class II medical device.
The latest development aids in the usage of the tube as an in-vitro diagnostic (IVD) device for Covid-19 testing.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The device is the first FDA-cleared technology that can inactivate the virus and preserve the SARS-CoV-2 RNA.
Effective inactivation of the SARS-CoV-2 virus permits the safe handling, transportation and storage of the sample, ensuring the safety of frontline healthcare and laboratory staffs.
The viral RNA is stabilised at ambient temperature for prolonged periods for robust analysis via downstream RT-PCR.
Zymo Research business development vice-president Dr Marc Van Eden said: “DNA/RNA Shield had a proven track record in various infectious disease applications prior to the current pandemic, facilitating its rapid adoption and deployment in the early stages of the Covid outbreak.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The 510(k) is the result of the FDA’s active collaboration with Zymo Research in bringing this technology to the forefront of current testing and future surveillance efforts.”
The product comprises a tube filled with the company’s proprietary DNA/RNA Shield transport medium, which ensures the SARS-CoV-2 RNA’s stability during specimen transportation and storage for up to 28 days at ambient temperatures.
The media may be kitted with a swab, sputum collection kit or as a tube alone.
Zymo Research noted that the technology is compatible with upper and lower respiratory specimens taken from people suspected of having SARS-CoV-2.
Furthermore, specimens collected and stored in a collection tube are well-suited for use with appropriate molecular diagnostic tests.
Last November, Zymo Research received the CE IVD mark for Quick-DNA/RNA Viral MagBead Kit for distribution to the European Union common market.
It was designed for high-throughput purification from biological samples that are stored in the company’s DNA/RNA Shield used for sample collection, nucleic acid preservation and inactivation of pathogens.





