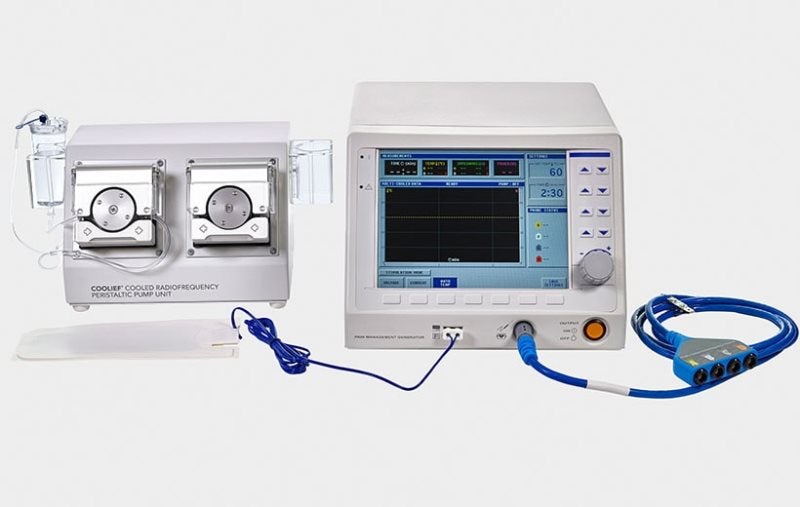
Avanos Medical has secured clearance from the US Food and Drug Administration (FDA) for marketing its 80W COOLIEF Radiofrequency (RF) system for neurological lesion procedures.
An easy-to-use system, it consists of peristaltic pump and therapy cables and a newly designed RF generator.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The 80W COOLIEF Radiofrequency (RF) system allows doctors to conduct a complete range of procedures including cooled, standard, pulsed, transdiscal and dual bipolar RF.
A minimally invasive outpatient pain management and a non-opioid procedure, Cooled RF leverages cooled radiofrequency energy to target the sensory nerves causing pain.
Around 100 million adults in the US suffer from chronic pain conditions, more than the number of adults affected by heart disease, diabetes and cancer combined.
Avanos claimed that many studies have shown that opioid usage for the treatment of chronic pain, may worsen pain and functionality.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOchsner Health system chair of pain medicine Dr Maged Guirguis said: “Avanos’ new Cooled RF system is intuitive and easy to use.
“The Cooled RF technology has not only helped our patients achieve significant relief from osteoarthritis pain, but it has also improved their function, thus providing a better quality of life.”
This system offers 60% more power compared to the legacy system. It comes with an intuitive touchscreen interface, and independent channel control, which allows doctors to start, stop and adjust on four probes individually.
Avanos CEO Joe Woody said: “For over a decade, Avanos has been committed to driving innovation and investing in clinical evidence in RF technology.
“The addition of our new, advanced COOLIEF RF generator demonstrates this continued commitment to introduce innovation to the marketplace as the Cooled RF authority and to provide relevant solutions that help patients effectively manage pain without the risk of addiction, so they can get back to things that matter. We’re excited to debut the new system at the upcoming American Society of Interventional Pain Physicians annual meeting.”
COOLIEF Cooled RF uses water-cooled technology, allowing RF energy to safely deactivate pain-transmitting sensory nerves. This leads to a more comprehensive, spherical lesion that distally projects 45% or more beyond the probe’s tip, enabling doctors to reach the target nerve from any angle.



