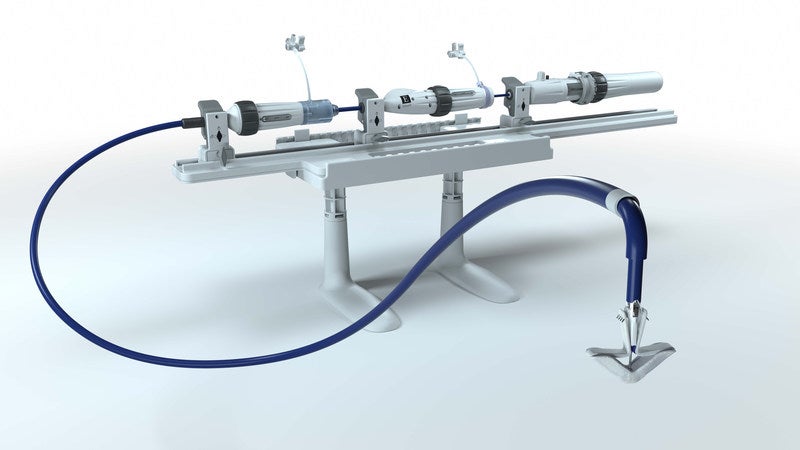
The US Food and Drug Administration (FDA) has approved the PASCAL precision transcatheter valve repair system from Edwards Lifesciences for the treatment of degenerative mitral regurgitation (DMR) patients.
The company has designed the system for transcatheter edge-to-edge repair (TEER) of the mitral valve.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
TEER grasps the anterior and posterior mitral valve leaflets using a clipping device, which is similar to a treatment known as the Alfieri stitch that was developed in cardiac surgery.
The PASCAL system has independent grasping, the ability to elongate and atraumatic clasp and closure features.
This allows it to provide safe and effective treatment for DMR patients.
The system also has an intuitive catheter and handle, which enables precise navigation and implant delivery.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEdwards transcatheter mitral and tricuspid therapies corporate vice-president Bernard Zovighian said: “Patients suffering with debilitating symptoms as a result of degenerative mitral regurgitation represent a large and significantly underserved group in the US.
“Edwards’ 60-year history of innovation and leadership within structural heart disease positions our team well to bring the PASCAL Precision system to US clinicians, supporting excellent real-world outcomes for patients.”
The company stated that the PASCAL precision system is one of its multiple transcatheter repair or replacement treatments that are in development and intended to treat mitral and tricuspid valve disease.
Edwards intends to present the data from the CLASP IID pivotal trial on 17 September at the 34th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation.
It is said to be the first randomised controlled trial that directly compared two contemporary TEER therapies.
Furthermore, Edwards noted that patients receiving treatment with the PASCAL precision system in the US will be enrolled in the TVT registry for five years.





