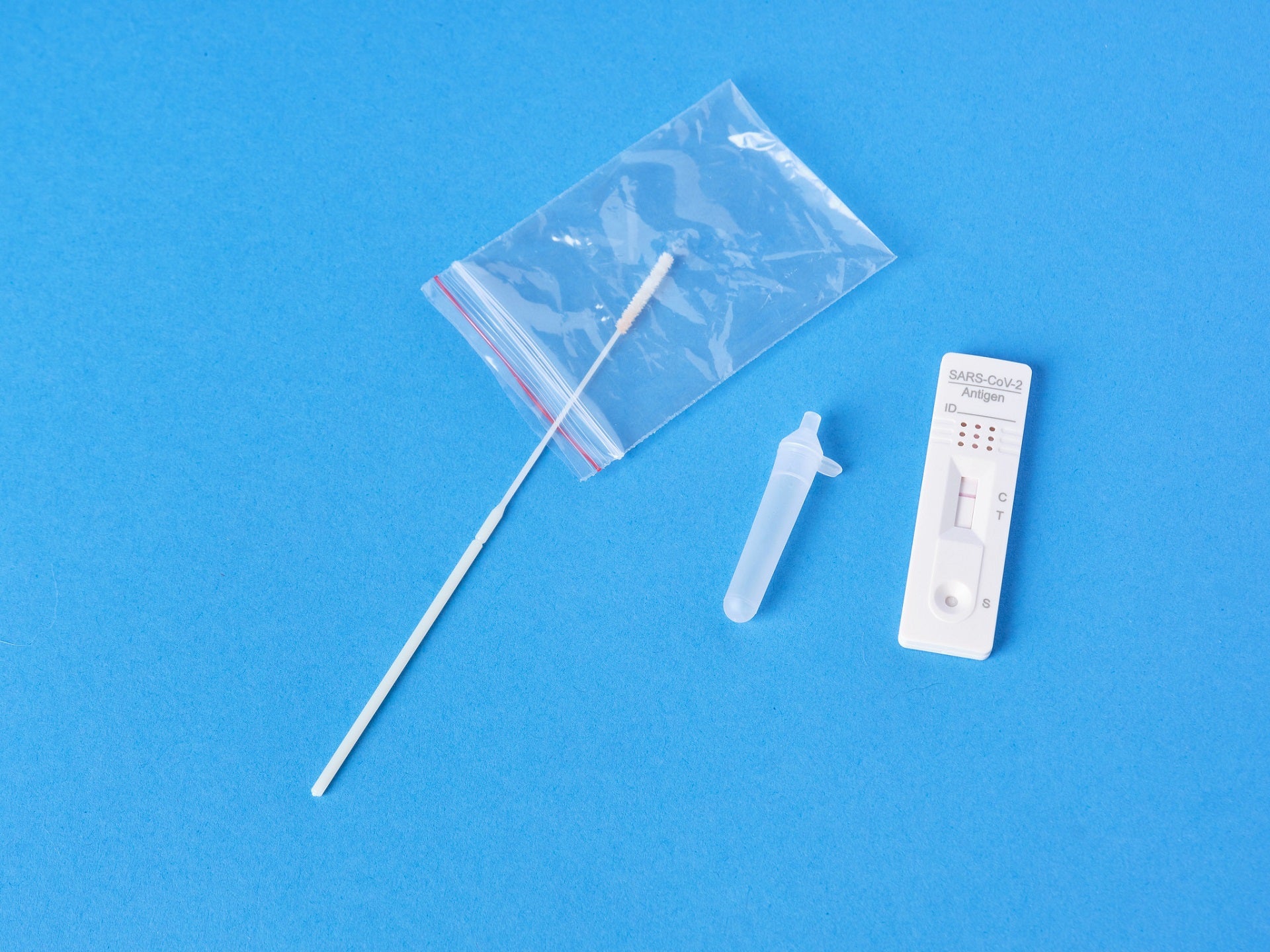
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) to Acon Laboratories’ Flowflex COVID-19 Antigen Home Test.
Flowflex is an over the counter (OTC) antigen test, which uses nasal swab specimens from individuals suspected of active Covid-19.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Individuals aged 14 years and above can use the new home test for self-testing. It can also be used for children aged two years or older with nasal swabs collected by adults.
Flowflex can currently be purchased without a prescription in major retail stores and will be available online soon.
The authorisation of the antigen test adds to the increasing list of Covid-19 tests that can be used at home without a prescription.
The FDA expects at-home Covid-19 testing capacity in the country to double over the next several weeks, as Acon plans to manufacture more than 100 million tests per month.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBy February next year, the number is expected to increase to 200 million each month.
The FDA also noted that Acon’s Flowflex home test does not require serial testing depending on the data provided for asymptomatic individuals.
US FDA Center for Devices and Radiological Health director Jeffrey Shuren said: “We believe at-home diagnostic tests play a critical role in the fight against Covid-19.
“We will continue to offer support and expertise to help with the development of appropriately accurate and reliable tests, and to facilitate increased access to tests for all Americans.”
Outside the US, the Acon’s Flowflex tests are already available in several countries, including extensive distribution in the UK through the National Health Service (NHS).





