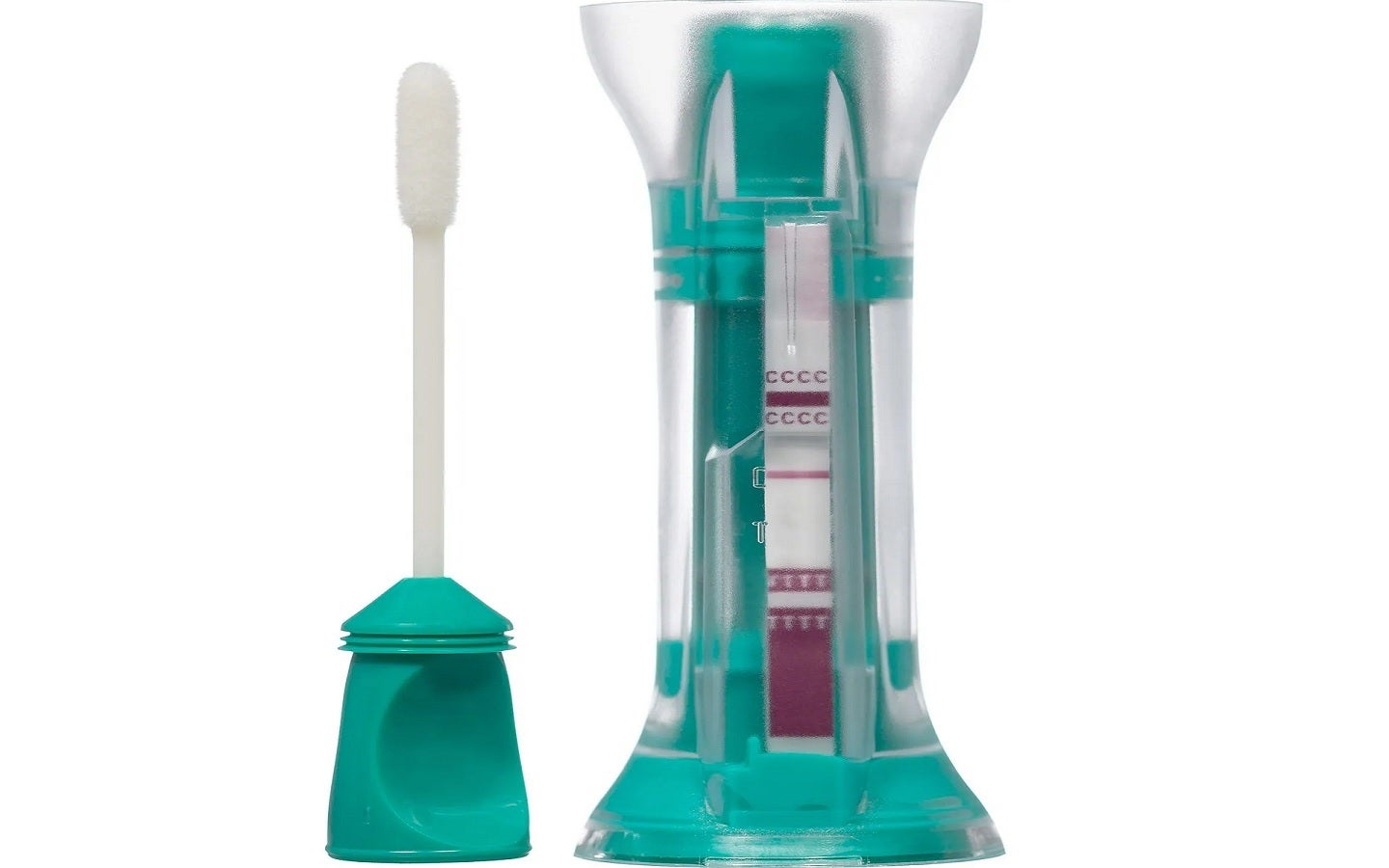
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) to the Mologic COVI-Go SARS-CoV-2 Ag Self-Test for over-the-counter home use.
The COVI-Go Covid-19 Self-Test has been designed for the qualitative detection of the SARS-CoV-2 virus nucleocapsid protein antigen in roughly 20 minutes.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is authorised for over-the-counter self-use by symptomatic individuals within the first five days of symptom onset, as well as for individuals without symptoms or other epidemiological reasons to suspect Covid-19.
The streamlined design of the new rapid antigen test includes an anterior nasal swab and test device.
Mologic stated that the all-in-one testing format of the COVI-Go test eliminates the need for mixing as well as the pouring of buffer solutions.
The self-contained unit of the COVI-Go SARS-CoV-2 Ag Self-Test is said to be safe and portable, with an integrated buffer that completely neutralises the Covid-19 virus in the sample to reduce biohazard risk and cross-contamination.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe US National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx) initiative provided funding for the development of the test.
Mologic plans to commercialise COVI-Go through collaboration with other organisations.
Global Access Diagnostics (GADx), Mologic’s parent company, CEO Mark Davis said: “Validation of the COVI-Go Self-Test by the FDA under EUA is a significant step in supporting the global fight against the threat of Covid-19.
“COVI-Go was made for everyday people and we look forward to supporting our partners in marketing its positive accessibility aspects, which also received positive feedback from accessibility groups like the Americans with Disabilities Act (ADA), highlighting its unique unitised design and easy-to-use functionality.
“As Covid-19 is here to stay alongside associated health challenges, the ability to leverage such a platform with the inclusion of additional respiratory diseases will be paramount to our continued strategy.”





