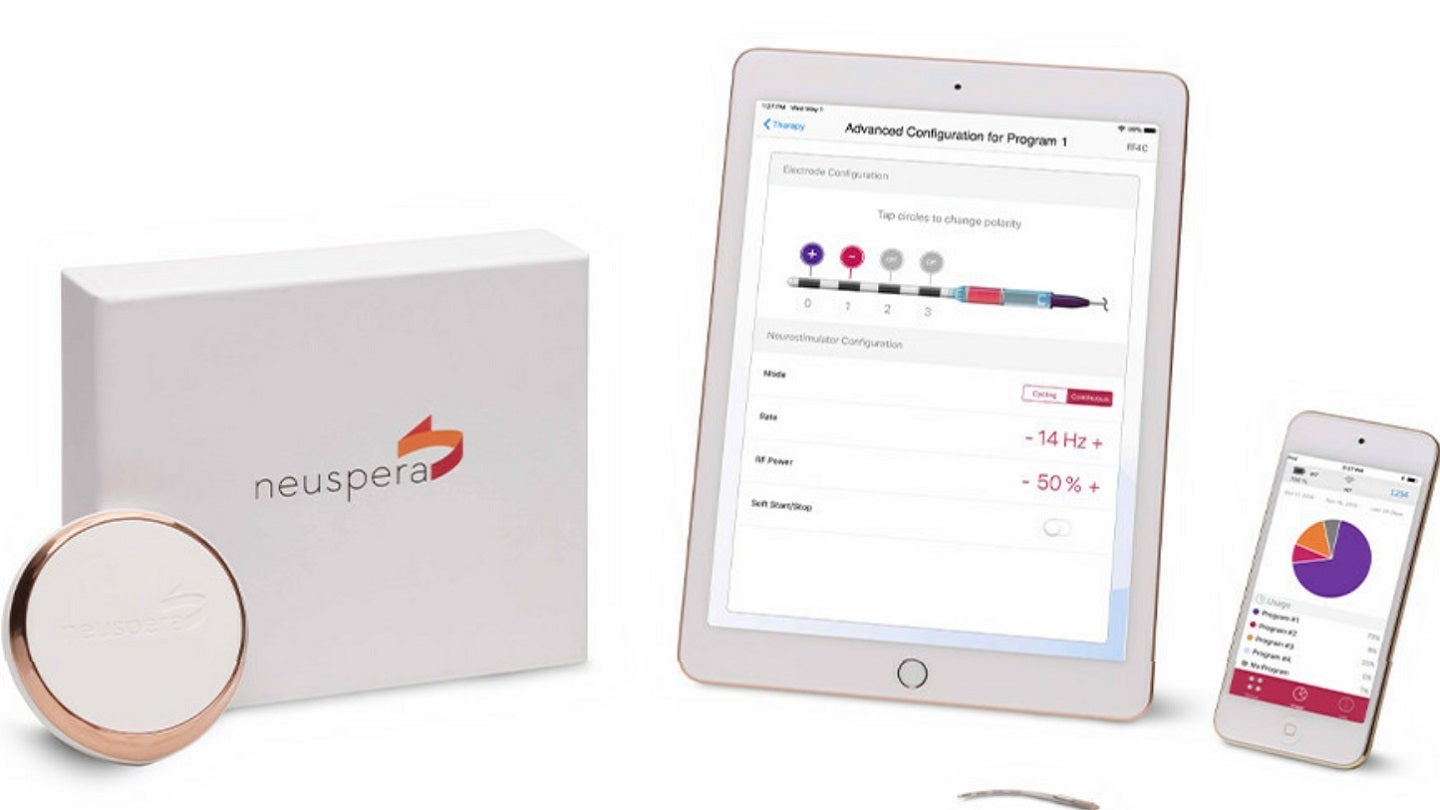
The US Food and Drug Administration (FDA) has approved medical device company Neuspera Medical’s next-generation ultra-miniaturised system for peripheral nerve stimulation.
The leadless micro-implant is designed for chronic peripheral nerve pain management. It offers neurostimulation therapy through a wireless platform, powered by a wearable transmitter.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The system also includes a smartphone app and an iPad-based clinician programmer which provides physicians with a command centre view of implanted devices and external wireless transmitters.
Neuspera Medical CEO Steffen Hovard said: “We look forward to bringing this innovative technology to physicians and patients in the US.
“The Neuspera ultra-miniaturised system has the potential to revolutionise the way physicians treat patients battling chronic pain while restoring patients’ health and quality of life.”
The Neuspera system provides an ultra-miniaturised option, which is expected to enhance patient experience and procedural flexibility.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPhysicians can use the device, which is claimed to be 75 times smaller than the smallest commercial implantable pulse generator, to reach deeper anatomical targets compared to other existing devices.
The Neuspera platform is intended to provide less-invasive and potentially earlier treatment options for physicians and patients.
In June 2022, Neuspera Medical unveiled plans to start the second phase of its SANS-UUI pivotal clinical trial of the Nuvella system.
The study is designed to evaluate the Nuvella system’s safety and efficacy to treat overactive bladder in patients with symptoms of urinary urgency incontinence.
Formerly known as Vivonda Medical, Neuspera is engaged in the development of implantable medical devices for patients with chronic illnesses.





