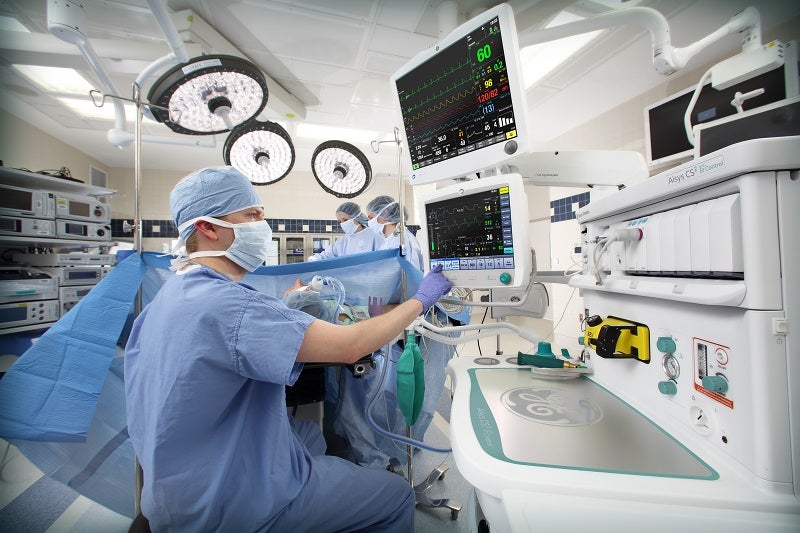
GE Healthcare has received pre-market approval (PMA) from the US Food and Drug Administration (FDA) for its End-Tidal (Et) Control software to deliver general anaesthesia.
The regulatory approval covers the use of the new software on the company’s Aisys CS2 Anesthesia Delivery System.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
GE Healthcare is claimed to be the only manufacturer in the US to get approval for providing general anaesthesia delivery with end-tidal concentration control.
Initially launched in Europe in 2010, the software semi-automates the anaesthesia delivery and allows anaesthesia providers to set targets for the end-tidal oxygen and anaesthetic agent.
After these targets are set, the Et control software will achieve and maintain them irrespective of changes in the hemodynamic and metabolic condition of a patient.
It improves the accuracy of anaesthesia delivery, simplifies the process, and reduces drug waste, greenhouse gas emissions and healthcare costs.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGE Healthcare anesthesia and respiratory care business general manager Eric Ruedinger said: “Anaesthesia providers in the US will have access to the most advanced anaesthesia tools available to improve patient care.
“As the long-standing global leader in anaesthesia delivery, GE Healthcare invested in the development and clinical validation of this Et Control algorithm, and we are committed to creating clinically relevant solutions that will enhance anaesthesia practices into the future.”
The regulatory approval was supported by data obtained from the multi-year, multi-centre MASTER-Anesthesia Trial, which was conducted on more than 200 subjects aged 18 years and above in the US.
In this study, the Et Control software’s effectiveness and safety were compared to conventional anaesthetic gas delivery methods.
The company plans to introduce the Aisys CS2 Anesthesia Delivery System with Et Control in the US in the upcoming months.





