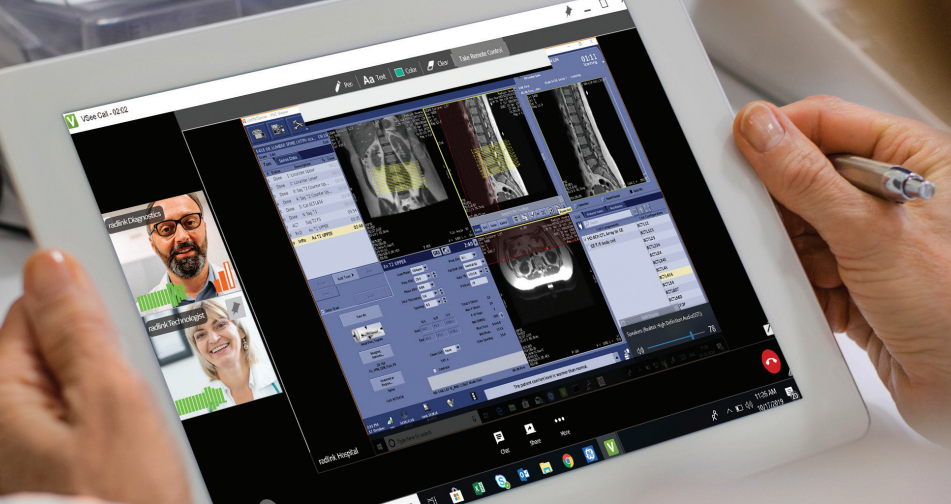
The US Food and Drug Administration (FDA) has cleared a new version of GE HealthCare’s Digital Expert Access – a virtual platform that connects radiology to discuss patient cases.
The new version can integrate remote patient scanning and enables a clinical expert to start a scan on compatible GE HealthCare magnetic resonance devices even when they are not in the radiology suite. It can be used to alleviate staff shortages to virtually bring in a trained, radiologist to conduct the scan.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
GE HealthCare Imaging CEO Jan Makela said: “With a shortage of radiologists, it is key for facilities to be able to leverage the skills of one expert across multiple physical locations.”
The global magnetic resonance imaging device market is estimated to reach $10bn by 2033, doubling from $5bn in 2022, according to a market model by GlobalData. GE HealthCare owns a 29.3% global market share, according to the model.
The platform still comes with the pre-existing features of enabling radiology teams across different hospitals or from within the same building to offer in-the-moment advice and remote control of consoles.
In the same statement announcing the 510(k) clearance, GE HealthCare said it has also entered into an exclusive distribution agreement with Brazil-based IONIC Health.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIONIC Health is the developer of the nCommand Lite system which can provide real-time guidance to the clinician operating the scanner. The device is currently under review by the FDA for 510(k) clearance.
GE HealthCare’s Imaging Platforms and Digital Solutions general manager Rekha Ranganathan said: “The overarching goal of the new version of Digital Expert Access and our collaboration with IONIC Health is to help healthcare systems increase efficiency and shorten the timeline to diagnosis for patients, which is a critical component in improving outcomes.”
Makela added: “The ability to seamlessly bridge multiple locations, users, and devices, enables technologists to share knowledge, improves exam workflows, speeds up decision-making and helps achieve clinical and efficiency goals.”





