The US Food and Drug Administration (FDA) has granted 510(k) clearance for HealthLytix’s breakthrough prostate imaging software, RSI-MRI+.
The approval leverages an advanced diffusion MRI technique known as Restriction Spectrum Imaging (RSI). It allows clinicians to detect and diagnose prostate cancer at an early stage.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
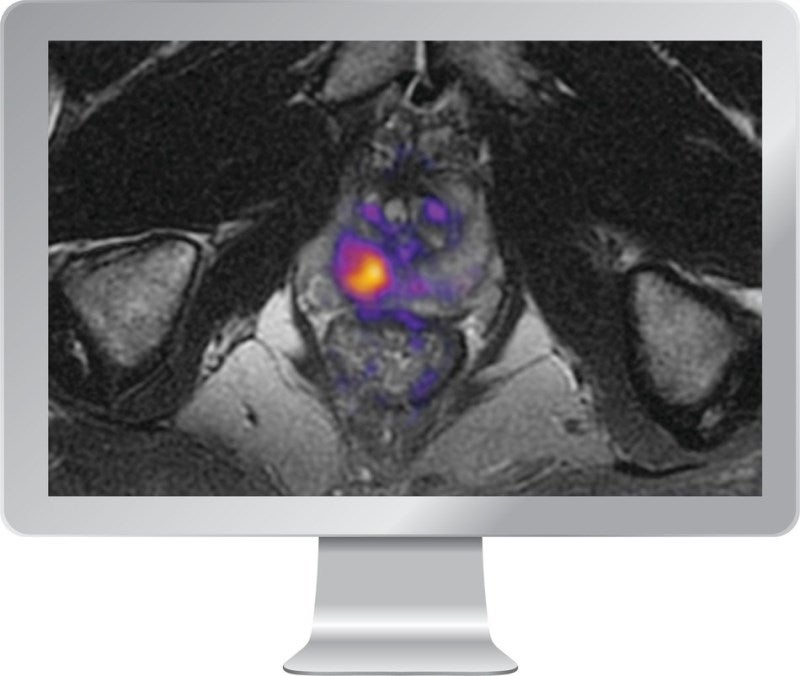
Claimed to be the first FDA-approved imaging software, RSI-MRI+ uses artificial intelligence (AI) and a tissue microstructure model to enhance the visibility of reduced water in the body’s tissue.
Reduced water is a hallmark feature of several cancerous tissues. The traditional diffusion-weighted imaging applies apparent diffusion coefficient (ADC) to calculate the limited water in the body’s tissue.
Various confounding factors pose a negative influence on ADC’s sensitivity and specificity to cancer, leading to missed or inaccurate diagnosis.
Contrary to the conventional diffusion-weighted imaging (DWI), HealthLytix’s RSI-MRI+ imaging software characterises the restricted signal component, Restricted Signal Map, to provide improved visualisation and quantification of reduced water.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHealthLytix CEO Nathan White said: “A key innovation of RSI-MRI+ is that it can better characterise the complexity of water diffusion in cancerous tissue, resulting in better performance. With early-stage aggressive cancer, we need more sophisticated approaches to separate what’s important from what’s not important.
“In cancer, that means separating restricted diffusion from other sources of water diffusion that are less relevant.”
Currently, the FDA has cleared RSI-MRI+ only for prostate cancer. In future, the company plans to secure FDA clearance for multiple body regions.
In December, HealthLytix will launch its RSI-MRI+ in the Radiological Society of North America (RSNA) annual meeting in Chicago.





