Italian medical device firm I-VASC has raised $1.95m (€1.8m) in a Series A financing round to launch VELEX, a device to treat chronic venous insufficiency (CVI).
The Luca Trevisan, Bootes (Rosario Bifulco), Nalini family Office and other qualified investors participated in the financing round along with former shareholders.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company intends to use the funding for post-market clinical studies of the VELEX device, to complete the industrialisation of the product and to obtain approval for the US market from the Food and Drug Administration (FDA).
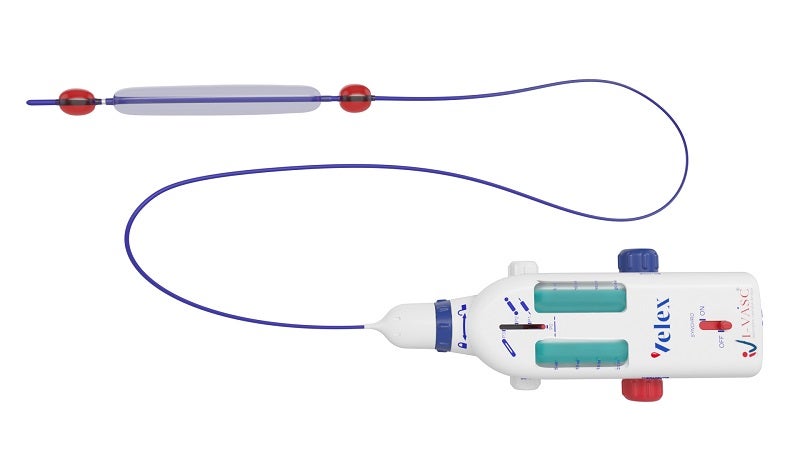
VELEX is a patented minimally invasive medical device that is developed for the empty vein ablation (EVA) procedure in CVI patients.
Claimed to be the company’s first CE Marked device, the non-thermal, non-tumescent endovascular device comprises a percutaneous three-ballon catheter that allows the performance of chemical ablation (Schlerotherapy) following isolation and removal of blood from the vein portion that needs to be treated.
I-VASC stated that this process enables full control of the schlerosant agent contact, distribution and time.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe newly appointed I-VASC CEO Daniele Zanotti said: “I am thrilled to embrace this new professional adventure and put my experience at the service of a project which has the potential of representing a new paradigm in the largely underserved market of CVI and varicose vein.
“With the considerable efficacy, safety and usability improvements that VELEX can offer with respect to all alternative methods, we have the opportunity to offer a better option to millions of patients and create a huge new value in the vascular arena.”
I-VASC raised $81,571 (€75,000) in the first half of last year, as well as another $1.159m (€1.066m) before the end of the year.





