US-based cell diagnostics company Ikonisys has signed a partnership agreement with Israel-based Sheba Medical Center to develop Circulating Tumor Cells (CTC) tests to detect and target specific cancers.
The tests and applications will be focused on addressing various clinical needs, including treatment monitoring and companion diagnostics.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Ikonisys will play a key role within Sheba Medical Center’s ARC, an innovation hub that accelerates global healthcare by collaborating with partners.
Sheba Medical Center Institute of Pathology head Iris Barshack said: “We are excited to begin using the Ikoniscope system provided by Ikonisys. Together, we aim to improve the detection of disease recurrence and clinically validate applications for various types of cancers while contributing to Sheba Medical Center’s mission to deliver excellent, highly innovative comprehensive diagnoses and care management to patients.”
Ikonisys chief scientific officer Michael Kilpatrick said: “We are proud to cooperate with one of the world’s leading medical centres in developing innovative tests for the diagnosis and monitoring of cancers.
“This partnership validates our proprietary Ikoniscope technology and coincides with the launch of our second-generation system. The cooperation also enables us to further expand Ikonisys’ product portfolio, strengthening our commitment in the world of CTCs to continuously improve cancer treatment.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe CTC detection test enables a clinician to detect, quantify and analyse tumour cells in the blood of patients suffering with cancer.
The applications of CTC analysis include determining the status of a particular disease, rate of disease progression and predicting the likely efficacy of a particular treatment.
With these capabilities, the CTC platform has the potential to change the landscape of cancer diagnosis. So far it has been restricted due to difficulties in detecting extremely rare cells that can be as few as ten out of millions in a typical 10ml blood sample.
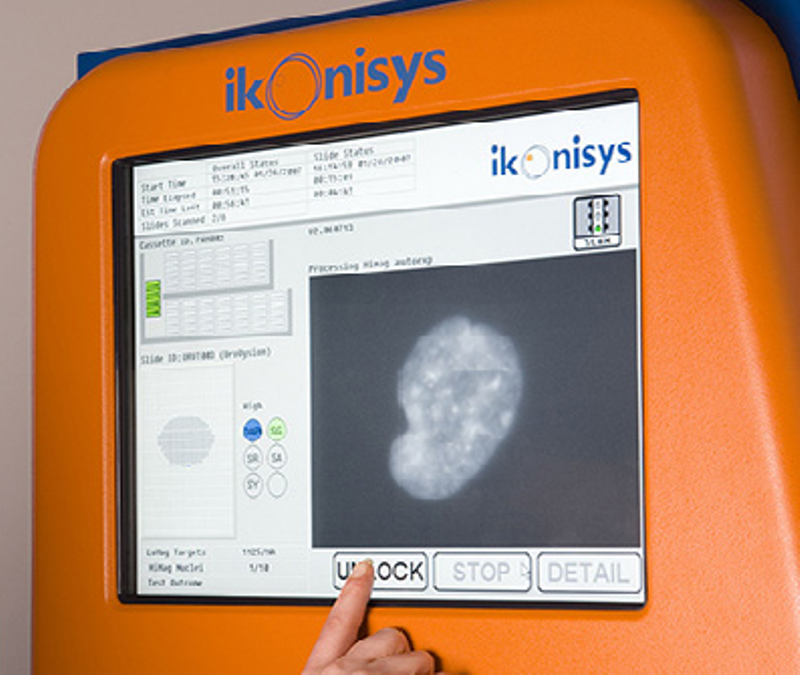
The Ikinoscope platform with its automated scanning and analysis capabilities can efficiently screen a much larger number of cells than manual analysis. This in turn allows identification and enumeration of CTCs in the blood of cancer patients with high specificity and sensitivity.
Ikoniscope is a robotic, high-throughput microscopy system. Alongside offering quality cell images, the platform evaluates cells of interest and provides an interpretation of the analysis, which in turn enables quick, accurate reporting of the test result.
In addition to automating conventional FISH-based tests, the rare cell detection and analysis capabilities of the platform helps to examine large complex samples for clinically-relevant cells. This capability makes it especially feasible for circulating tumour cell-based testing. The next generation of the system launched with this project offers improved performance as it comes in minimal size and weight and a modular design, thereby enabling laboratory-specific customisation.
Led by Prof Iris Barshack, Sheba’s research team will identify and propose biomarker panels for particular cancers to be evaluated as potential Ikinoscope CTC tests.
The team will collect available clinical data from patient samples, which will allow analysis of the potential complementary nature of cell-based CTC tests.





