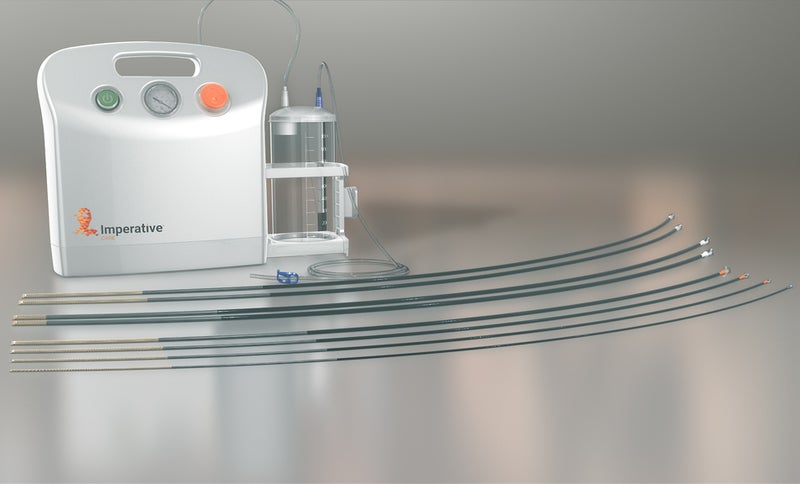
Medical technology company Imperative Care has received 510(k) clearance from the US Food and Drug Administration (FDA) for the use of its Zoom aspiration system to remove clots in ischemic stroke patients.
The aspiration system comprises Zoom reperfusion catheters, Zoom aspiration pump, Zoom canister, and the Zoom aspiration tubing.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Zoom reperfusion catheters feature the TRX tip to navigate complex anatomy and allow smooth tracking of blood clots in the brain’s tortuous vasculature. They are available in four diameter sizes of .071in, .055in, .045in and .035in.
The flexible catheters are said to complement the firm’s access catheters range intended for consistent navigation deep into the blood vessels of the brain.
Imperative Care marketing executive vice-president Ariel Sutton said: “For any ischemic stroke procedure, getting to the brain quickly with the right tools to remove clots is paramount. Imperative Care has developed a portfolio of access and aspiration catheters that work together to facilitate fast and effective clot removal.
“Imperative Care is laser-focused on improving stroke management and committed to developing technologies that speed solutions to stroke across the full continuum of care. The availability of these products brings us one step closer to making that goal a reality.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe new aspiration system can be used for the revascularisation of patients who have acute ischemic stroke secondary to intracranial large vessel occlusive disease within eight hours following symptom onset.
Imperative Care noted that patients not eligible for intravenous tissue plasminogen activator (IV t-PA) or those who failed IV t-PA therapy can be treated with the Zoom system.
The company intends to roll out its portfolio of products over the coming months.
Imperative Care is the 21st start-up established by medtech entrepreneur Fred Khosravi through a medical technology accelerator and development firm called Incept.





