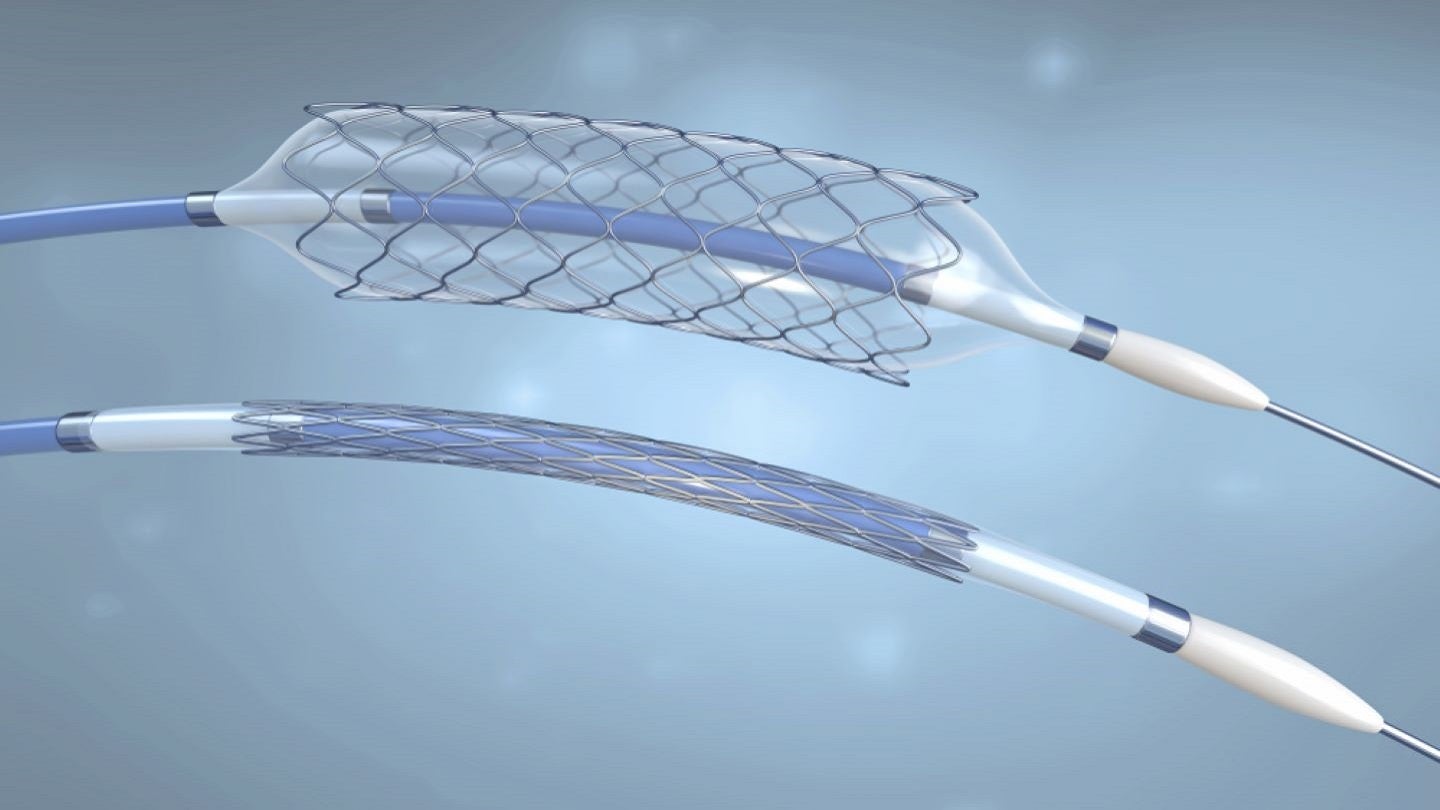
Medical device company Inari Medical has launched two new purpose-built thrombectomy catheters to address the needs of venous stent thrombosis and venous thromboembolism (VTE).
The two products are the Triever16 Curve catheter and the RevCore thrombectomy catheter.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Part of the FlowTriever platform, the Triever16 Curve catheter is designed for use in both peripheral thrombectomy and pulmonary embolism procedures.
Triever16 Curve is a highly trackable catheter equipped with a pre-shaped curve for targeted aspiration and it further provides distinct benefits compared to 16F continuous aspiration catheters.
The advantages include its compatibility with the FlowSaver blood return system and convenient accessibility to larger and more potent 20F or 24F catheters.
On the other hand, RevCore is claimed to be the first mechanical thrombectomy device developed to treat venous in-stent thrombosis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe device comes with an advanced diameter-controlled coring element specially created to safely engage thrombus within stents.
Through the RevCore product, Inari can obtain access to new patients who are not currently treated by the FlowTriever or ClotTriever platforms.
Inari CEO Drew Hykes said: “We are thrilled to announce the commercial launch of these two products, which reinforce our unwavering commitment to addressing unmet needs with purpose-driven innovation.
“We continue to push the boundaries, and we are excited about the potential impact we can make on patients’ lives with these products and beyond.”
Last month, Inari announced planned recruitment for its third randomised controlled trial (RCT) in VTE to assess its FlowTriever system.
PEERLESS II is a multi-centre, prospective, global RCT designed to compare the clinical outcomes of the FlowTriever system against anticoagulation alone in intermediate-risk pulmonary embolism patients.





