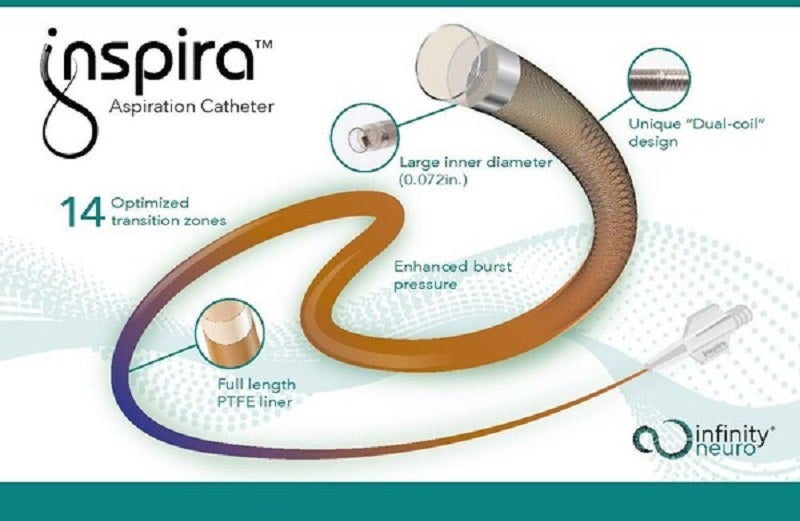
Medical device company Infinity Neuro has received CE Mark approval for its Inspira aspiration catheters to treat stroke.
The new Inspira aspiration catheters have been designed for easily evaluating target vessels, restoring cerebral blood flow quickly, and retrieving clots causing large vessel occlusion (LVO) and acute ischemic stroke (AIS) in patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
They are developed to provide next-level navigation and aspiration performance.
The catheters are indicated to inject intravascular fluids and introduce interventional devices into the peripheral and neuro vasculature.
They are currently available in Europe.
Additionally, they can also be used for soft emboli and thrombi removal/aspiration from the arterial system, including peripheral and neuro vasculature.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company stated that the new neurovascular device Inspira is the first phase of a complementary integrated system that has been developed to offer ease of use and better performance with first-to-market product innovations.
Inspira aspiration catheters are Infinity Neuro’s first offering. The company intends to introduce a complete range of products to treat ischemic and haemorrhagic stroke over the next few years.
The latest move represents the company’s commitment and ability to attain class III medical device approvals through the new European MDR CE certification process.
The Inspira aspiration catheters represent the first neurovascular device to receive approval under the new European MDR CE certification process.
The commercial launch of Inspira aspiration catheters in Europe is currently underway in collaboration with an EU distributors network.
According to the company, stroke is the third-leading cause of combined death and disability, as well as the second-leading cause of death globally.





