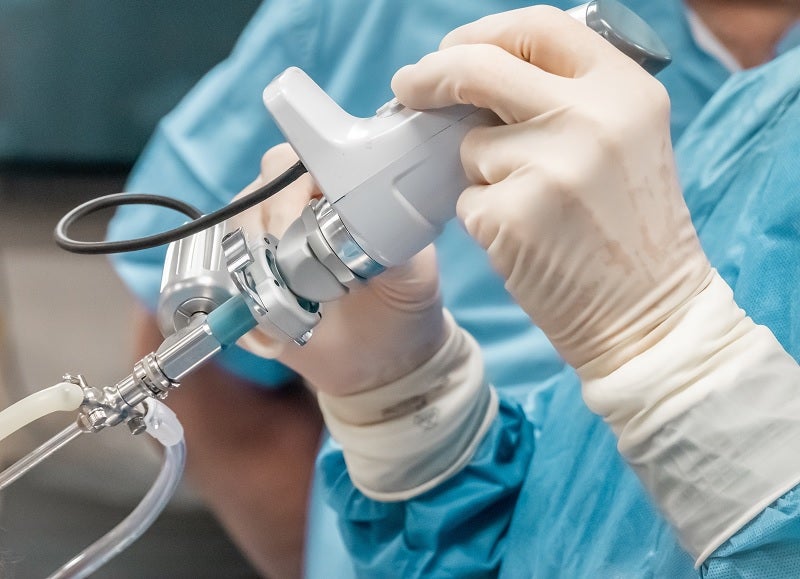
Medical device start-up Lazurite has received US Food and Drug Administration (FDA) market clearance for its ArthroFree wireless surgical camera system for minimally invasive surgery.
The modular system comprises Lazurite’s high-intensity Meridiem light technology along with a battery, modern camera and wireless communication technologies.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has also been designed to have drop-in compatibility with patient data consoles, endoscopes and surgical displays.
Using the ArthroFree System, surgical visualisation in minimally invasive surgery is possible without the need for camera and light wires.
Lazurite Board chair Mark Froimson said: “We are proud to have achieved this major milestone on our way to bringing operating rooms across the country into the wireless age.
“In the coming year, we will be introducing the ArthroFree System to thousands of healthcare professionals at conferences, in demos, through distributors and group purchasing channels, and through partnerships with leading medical centres.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company stated that the device has been developed to offer patient safety, improved operating room productivity, and economic value through energy efficiency, cost-savings, and lower set-up and breakdown times.
Lazurite CEO and co-founder Eugene Malinskiy said: “The idea for what is now the FDA-cleared ArthroFree System was born from the very real need to create a safer, more efficient operating room for the benefit of everyone involved – from patients to surgeons to OR teams to clinics and hospitals.”
“The entire endoscopic market will benefit from our minimally invasive surgical equipment advancement going forward.”
Designed to simplify sterilisation as well as minimise set-up and take-down times, the ArthroFree System eliminates hazards caused by fibre-optic cables.





