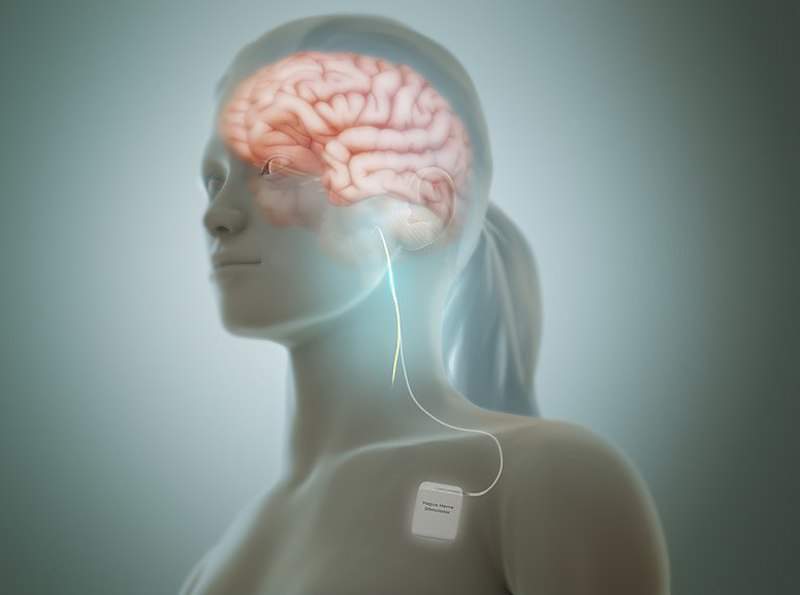
UK-based medical technology firm LivaNova has started implanting patients in the ANTHEM-HFrEF pivotal study with its Vitaria System for treating advanced heart failure.
The Vitaria System is designed to deliver autonomic regulation therapy (ART) through vagus nerve stimulation (VNS). The device is intended for patients who experience symptoms of heart failure even after guideline-directed medical therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In 2015, the system secured the European CE-Mark, while the US Food and Drug Administration (FDA) granted expedited access pathway designation last year.
ANTHEM-HFrEF is an international, multi-centre, randomised study designed to assess the safety and efficacy of autonomic regulation therapy delivered by the Vitaria System in around 800 subjects.
During the trial, the device will be implanted on the right cervical vagus nerve to deliver continuous, periodic VNS stimulation.
The first implant was carried out at UnityPoint Health – St. Luke’s Hospital in Cedar Rapids, Iowa, US.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLivaNova Neuromodulation general manager Edward Andrle said: “Autonomic regulation therapy is based on our market-leading experience derived from VNS Therapy, which is used to treat other disorders.
“The ANTHEM-HFrEF pivotal study brings us closer to offering the Vitaria System to a large segment of the more than 20 million people currently battling heart failure around the world.”
According to the company, Vitaria may be the first implantable active neurostimulation system intended for advanced heart failure treatment if the system displays statistically and clinically meaningful improvements in certain pre-specified endpoints of heart failure symptoms, hospitalisation and survival during the study.
The pivotal trial is expected to be completed in December 2022.





