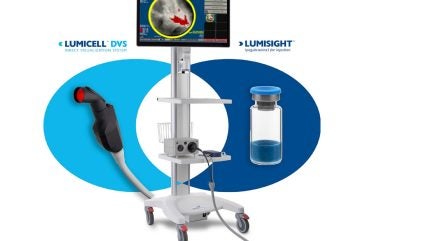
Lumicell has received approval for its new drug application (NDA) for LUMISIGHT optical imaging agent and premarket approval (PMA) application for Lumicell direct visualisation system (DVS) from the US Food and Drug Administration (FDA).
The LUMISIGHT optical imaging agent and Lumicell DVS are together referred to as LumiSystem, which aims to assist surgeons in detecting residual cancer cells during breast cancer surgeries.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Lumicell said the LumiSystem demonstrated an 84% diagnostic accuracy in identifying cancerous tissue that might have been missed during lumpectomy surgery and sparing some patients from second surgeries.
The LumiSystem is indicated for fluorescence imaging in adult breast cancer patients as an adjunct tool for intraoperative detection of cancerous tissue within the resection cavity after the removal of the main specimen during the surgery.
The system’s safety was established using data from more than 700 breast cancer patients across five clinical studies at leading academic and regional community cancer centres in the US.
The most commonly found side effects with the use of LUMISIGHT included hypersensitivity and urine discolouration, with serious hypersensitivity reactions, including anaphylaxis, also being a possibility.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataResults from the Investigation of Novel Surgical Imaging for Tumor Excision (INSITE) pivotal trial, published in NEJM Evidence, supported the efficacy of the system.
Lumicell president and CEO Howard Hechler said: “We are immensely proud of the dual approval of LUMISIGHT and Lumicell DVS – we believe this is the first drug-device combination product approved in over a decade to have followed both of the FDA’s most stringent NDA and PMA review processes.
“With the FDA’s approval, LumiSystem is now the first and only imaging combination product capable of detecting cancerous tissue where it matters most, inside the breast cavity.”
Earlier, LUMISIGHT and Lumicell DVS secured FDA Fast Track and Breakthrough Device designations, respectively.
Last May, the FDA accepted the PMA application for Lumicell’s breast cancer detection assistance device.
………………………….
PR/Image
Image





