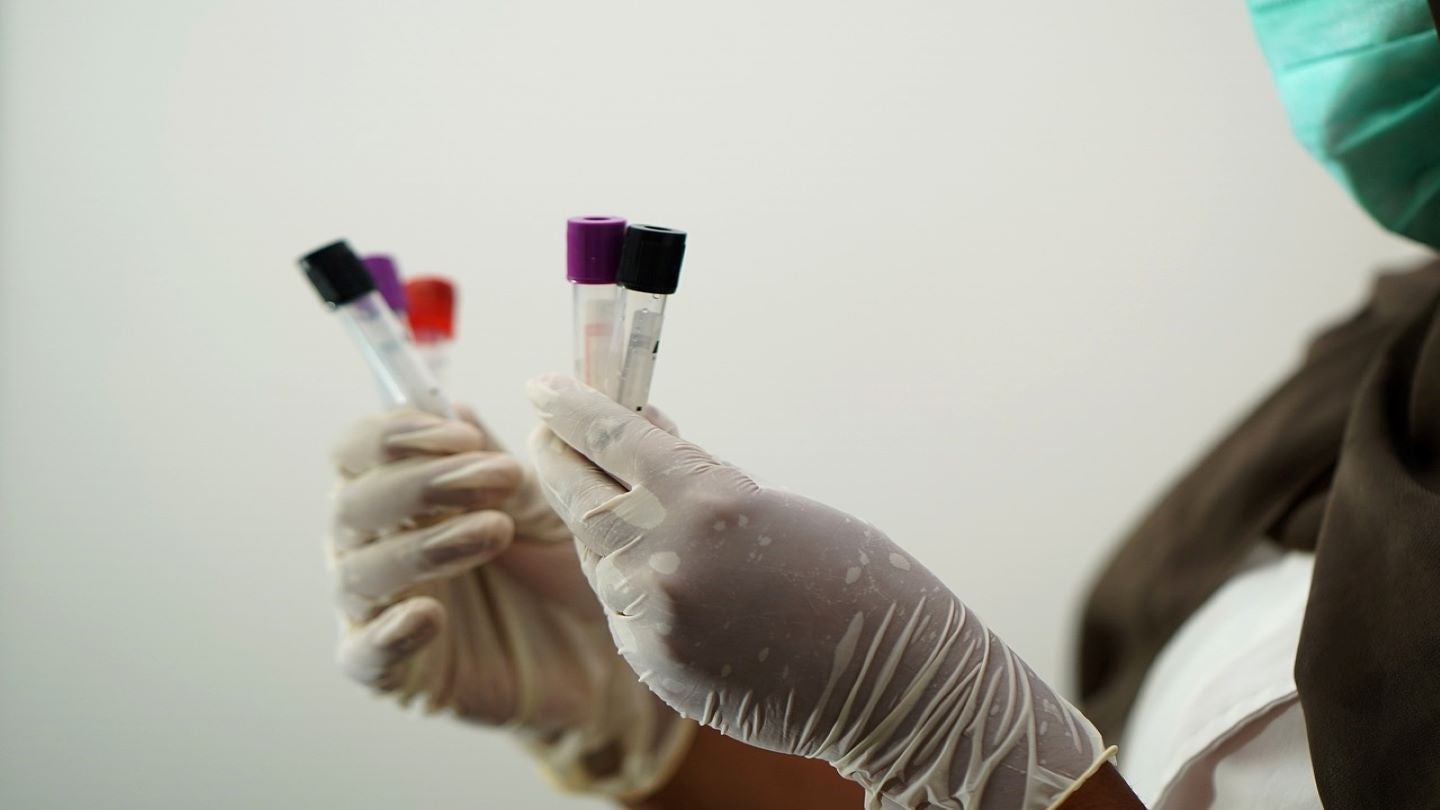
Maternova has entered a multinational distribution agreement with MedMira to market rapid diagnostics for human immunodeficiency virus (HIV), syphilis and hepatitis.
As part of the agreement, Maternova will exclusively supply MedMira’s Reveal, Multiplo and Miriad brands across Latin America.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Developed to reduce HIV, hepatitis and syphilis as well as other infectious and sexually transmitted diseases, the company will also have non-exclusive rights for the products in the US.
Maternova founder and strategy chief Meg Wirth said: “Sexually transmitted infections are a growing threat to maternal and infant health.
“MedMira’s Rapid Vertical Flow platform provides tests with immediate results that are highly sensitive and specific, all of the qualities needed by clinicians.”
Designed to reduce mother-to-child transmission (MTCT) of syphilis and HIV, the Multiplo TP/HIV rapid test is claimed to deliver 100% sensitivity in detecting co-infections.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to data from recent studies carried out by the Centers for Disease Control and Prevention (CDC), the Reveal G4 HIV rapid test is also claimed to offer the highest sensitivity and specificity.
These products are exclusively manufactured at a facility in Canada that is US Food and Drug Administration (FDA) and Medical Device Single Audit Program (MDSAP) certified.
MedMira CEO Hermes Chan said: “MedMira has developed a number of highly valuable and essential rapid tests to provide immediate quality answers in any setting.
“Our core focus is the development and manufacturing of our technologies and products, and we are delighted to partner with Maternova to access new markets and new customers.”





