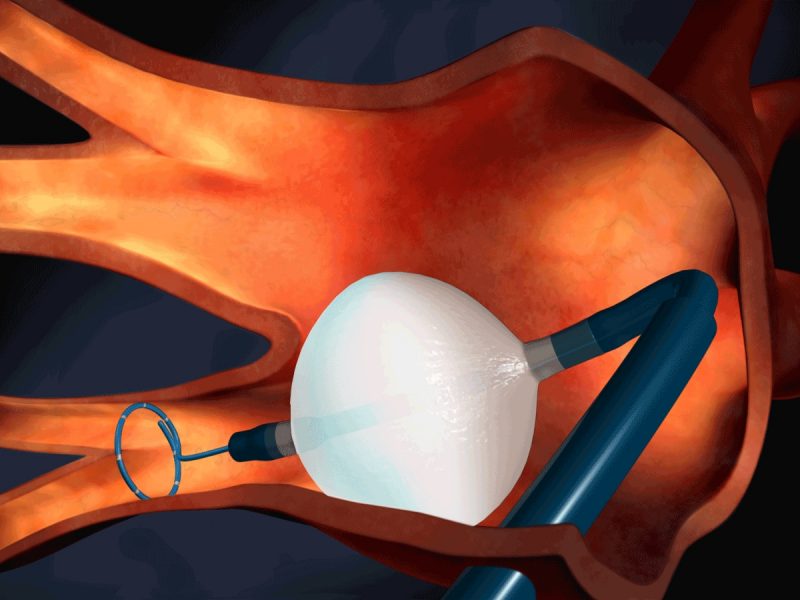
Medtronic has obtained expanded approval from the US Food and Drug Administration (FDA) for its Arctic Front Family of Cardiac Cryoablation Catheters to treat recurrent symptomatic paroxysmal atrial fibrillation (AF).
The catheters are intended to be used as an early rhythm control approach for paroxysmal atrial fibrillation episodes that last less than seven days.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
They can be used as a substitute for antiarrhythmic drugs (AAD), Medtronic noted.
The FDA decision to expand the indication is based on positive data from a prospective, multi-centre, randomised STOP AF First trial, which enrolled 225 subjects at 24 sites across the US.
According to results from the study, Medtronic’s cryoablation procedure showed greater efficacy in preventing atrial arrhythmia recurrence as against AAD therapy.
Nearly 74.6% of subjects in the cryoballoon arm had successful treatment at one year versus 45% in the AAD group.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFurthermore, a reduced rate of patient complications was observed with catheter ablation procedure as first-line therapy.
Medtronic noted that the Arctic Front family of Cardiac Cryoablation Catheters are the first catheter ablation devices to receive approval in the US to aid doctors in enhancing AF patient outcomes before drug failure.
Furthermore, these catheters reduce the time from diagnosis to ablation with outcomes that are effective and predictable.
Medtronic cardiac ablation solutions business president Rebecca Seidel said: “With this milestone announcement, Medtronic now has the only ablation catheter approved by the FDA to be used as first-line treatment in the US to treat AF.
“The indication expansion demonstrates how Medtronic continues to lead the way in cardiac ablation solutions for arrhythmia management and fill a market need for an early rhythm control strategy for what is a very progressive disease.”
Earlier, the FDA extended the indication for the company’s cryoablation therapy to include people with drug-refractory recurrent symptomatic paroxysmal as well as persistent atrial fibrillation.
The development comes after Medtronic decided to discontinue the supply and marketing of its HeartWare ventricular assist device due to a high incidence of neurological adverse events.





