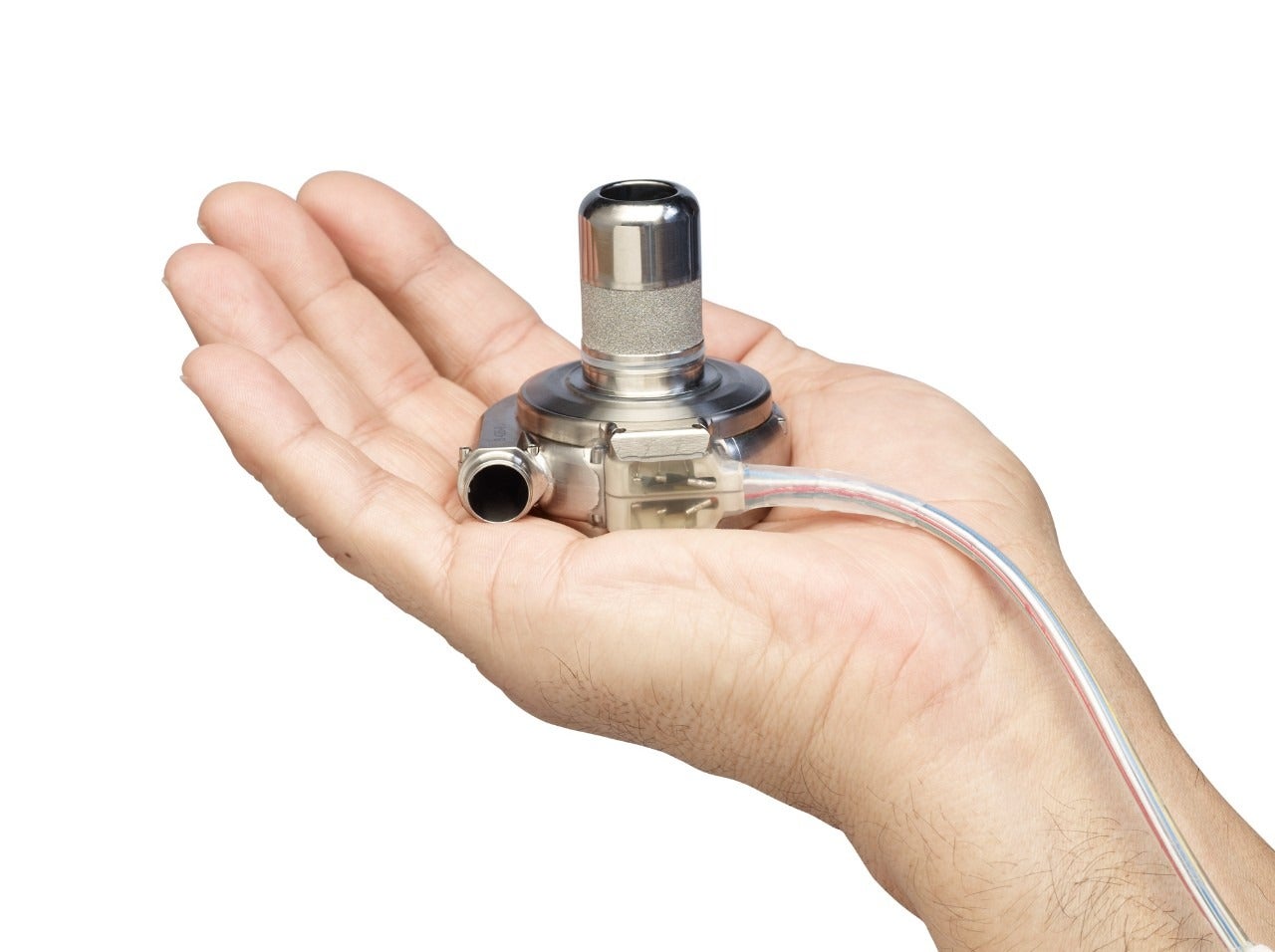
The US Food and Drug Administration (FDA) has issued a Class I recall of Medtronic’s HeartWare Ventricular Assist Device (HVAD) Pump Implant Kit, following several complaints that the device may fail to start, restart or have a delayed start after the pump was stopped.
These delays or failures to start or restart have occurred during pre-implant testing, during implantation and in a variety of post-implant situations.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The issue does not affect the pump while it is running, but can occur when the pump is stopped, for example during a controller exchange, and then restarted, Medtronic told Medical Device Network.
In a statement, the FDA said: “If the device has delays or fails to start or restart, this could cause serious patient harm including a heart attack, worsening heart failure, the need for additional procedures and hospitalisations, or death.”
A Medtronic spokesperson said: “An internal pump component from three specific lots puts a subset of the pumps at higher risk of this issue. Worldwide, 506 HVAD Systems were manufactured and distributed between 2017-19 with the impacted components.”
Of the 29 complaints made about this issue, two deaths have been reported, while 19 complaints detailed serious injuries and a further eight patients had a resulting life-threatening event but made a full recovery.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMedtronic has now recalled 157 devices from across the US.
The firm issued urgent communications to all affected customers in December, advising those fitted with the implants on how to avoid unnecessary pump stops and manage controller exchanges.
The HVAD Pump Implant Kit is part of the HeartWare HVAD System, which is used to help the heart pump blood around the body.
The device is used as a bridge to cardiac transplants in patients who are at risk of death from end-stage left ventricular heart failure, for heart tissue recovery, or as destination therapy in patients where new transplants are not planned.
This is the third Class I recall related to the HeartWare suite in the past 12 months.
In March 2020, the FDA issued a recall notice about the potential for users to insert the battery charger adapter into the wrong port after a patient death.
The agency issued a separate Class I recall in May about an outflow graft problem linked to four deaths.
Medtronic acquired HeartWare for $1.1bn in 2016, adding the HeartWare HVAD System to its portfolio.





