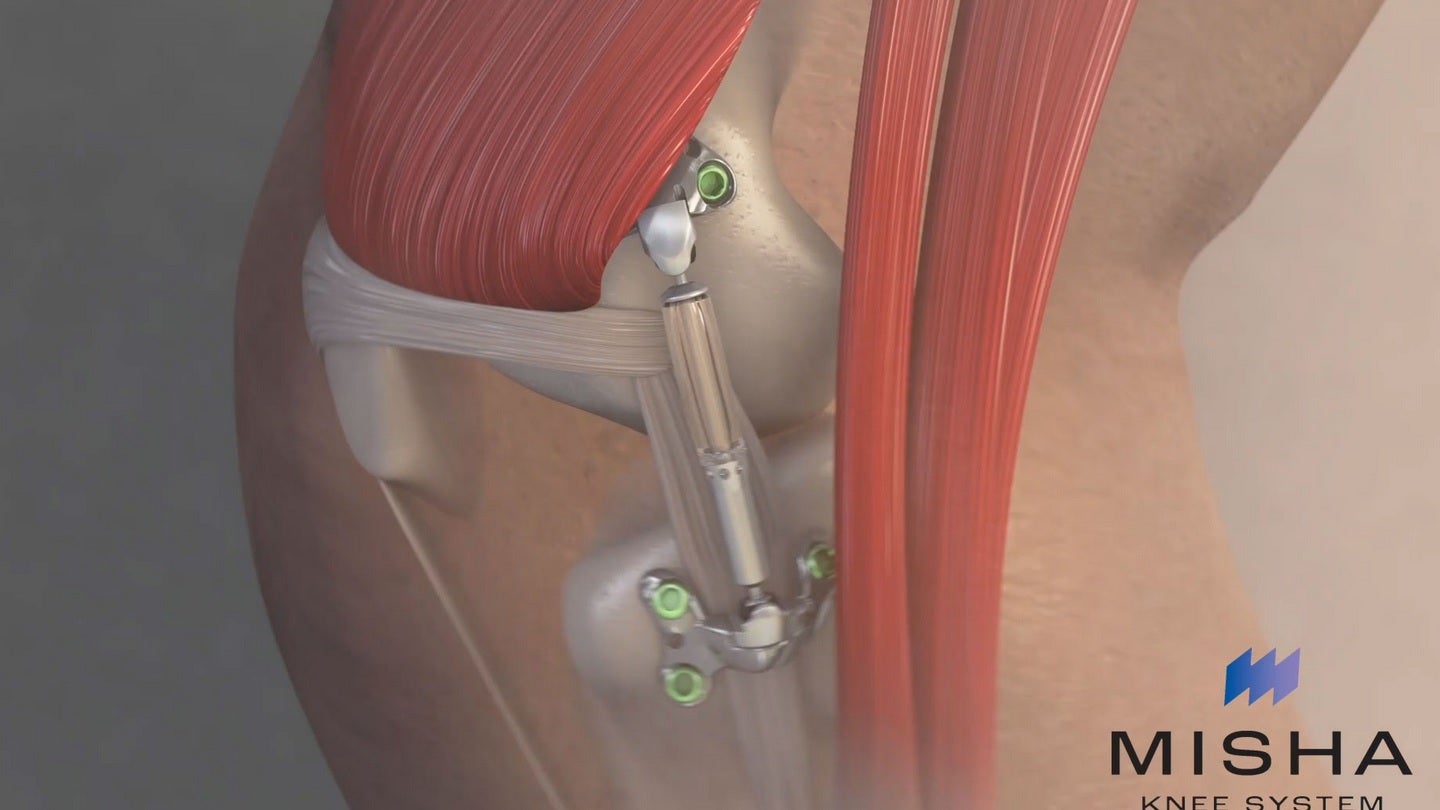
Medical device company Moximed has received marketing authorisation from the US Food and Drug Administration (FDA) for its MISHA Knee System.
MISHA is an implantable shock absorber (ISA) intended for the treatment of people with medial knee OA. This is a common and debilitating condition that affects more than 32 million people in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Moximed founder and CEO Anton Clifford said: “This is a milestone event for knee OA sufferers and it’s the result of unwavering clinical research and development that spans more than ten years.
“We offer special thanks to our study patients and surgeon investigators who helped advance the understanding of this new treatment for OA.
“Also, we recognise the dedicated reviewers at FDA for completing their thorough benefit-risk assessment of our breakthrough technology.”
The MISHA system is indicated for people who continue to experience pain despite undergoing non-surgical or surgical treatment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt is also suitable for patients who are not recommended to undergo joint replacement surgery due to age or the absence of advanced OA.
To develop the MISHA Knee System, the company applied the benefits of weight load reduction on diseased joints.
When implanted during an outpatient-compatible procedure, the system showed superiority over high tibial osteotomy.
William Jiranek, former president of the American Association of Hip and Knee Surgeons, said: “In my current role as vice-chair for Practice Innovation at Duke University, I consider treatments for OA across the entire disease continuum, rather than focusing only on the end stage.
“As such, I was invited to provide an independent, non-investigator assessment of Moximed’s clinical study protocol, and I provided an independent, periodic review of the data during the study.”





