MYND Life Sciences has announced the incorporation of a fully-owned subsidiary, MYND Diagnostics, to market biomarker diagnostic tests for patients suffering from various psychiatric disorders.
The diagnostic biomarker division of the company has a strong experimental pipeline of diagnostics, new small molecules and biologics.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The portfolio aims to improve outcomes of diseases as well as monitor and enhance the therapy experience in patients with major depressive disorder (MDD), treatment resistant depression (TRD) and various other inflammatory disorders of the central nervous system.
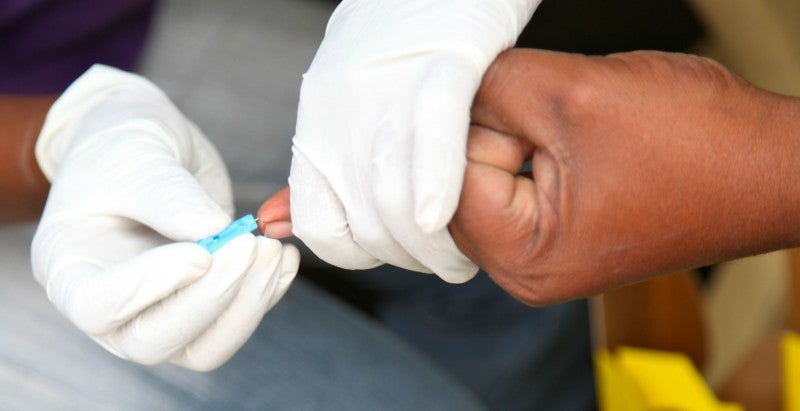
Facilitated by proprietary technology, the newly formed subsidiary’s innovation permits measuring of the MYND anti-inflammatory peptide (MAP) biomarker quantitatively and qualitatively.
MYND Life Sciences CEO Dr Lyle Oberg said: “For decades, the diagnosis of psychiatric illness relied almost exclusively on self-reporting and physician observation of symptoms to make a diagnosis.
“The MAP Biomarker will give healthcare providers an objective monitoring tool that will detect and diagnose mental illness earlier, thereby enabling quicker, more targeted treatments.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We will deliver an accurate indicator of depression, therefore allowing for better monitoring of improving or relapsing disease.”
MYND Diagnostics will work on carrying out clinical trials to detect a marker that can be used to quantitatively diagnose and monitor depression in response to therapy.
MYND noted that the biomarker market is undergoing quick growth which is attributed to the rising usage in discovery and development of drugs, diagnostics, risk analysis of target conditions and use in customised medications.
MYND Diagnostics possesses a competitive benefit as its process is cost-efficient and time-saving.
In addition, these tests can deliver results directly from a dried blood sample in comparison to the ones which require vials of whole blood.





