US-based Nanomix has secured CE mark for its S1 Assay for the quick detection of life-threatening infections such as sepsis and bacteremia.
Designed for use with Nanomix elab analyser, the CE-marked S1 Assay Panel will aid in rapid, simultaneous detection and quantification of lactate (LAC), procalcitonin(PCT) and C-reactive protein (CRP) in human plasma specimens.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is challenging to detect serious infections from clinical symptoms and delay in treatment raises the risk of death from sepsis.
The S1 Assay is claimed to be a simple, accurate and cost-effective panel that runs on the eLab analyser, intended to provide results of LAC, PCT and CRP within 11 minutes. It provides critical diagnostic information to physicians to offer appropriate decision-making treatments during diagnosis.
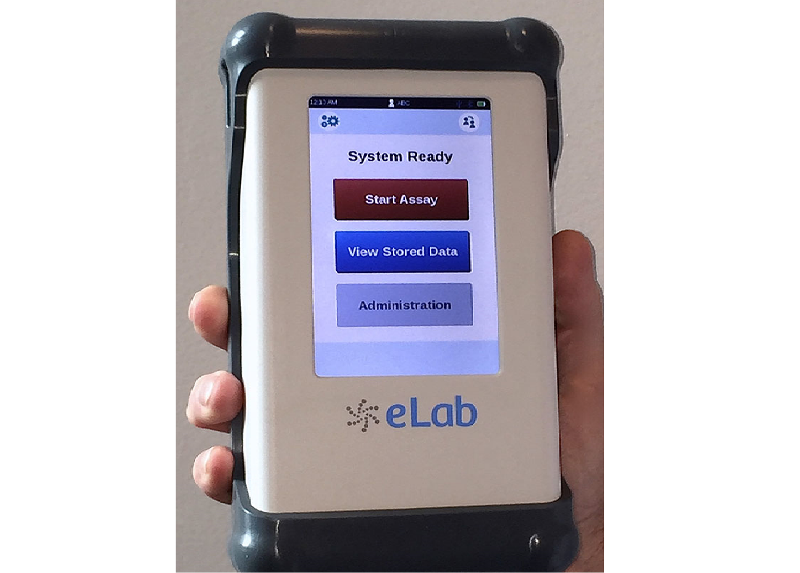
The Nanomix eLab analyser, suitable for use within or outside traditional laboratory settings, is a microfluidic-based handheld system, single-use consumable along with an electrochemical sensor for automated and simultaneous detection of several analytes.
In addition to Bluetooth and USB connectivity facilities, the eLab analyser features touchscreen graphical interface with a built-in barcode scanner.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataNanomix is currently evaluating a complete blood version of the test.
Nanomix president and CEO David Ludvigson said: “This novel assay increases the relevant information available to clinicians in the evaluation of serious infections when urgent decision-making is required. The Nanomix S1 Assay is the first multiplex product to address the diagnosis of life-threatening infections at the initial point of care.”
The company expects the S1 Assay panel and eLab instrument to be available through distribution within CE-regulated markets by next year.
The firm expects to submit the product to the US Food and Drug Administration (FDA) next year.





