Neuronoff, a medical device company developing minimally invasive neuromodulation solutions, has announced the completion of a first-in-human clinical trial for its Injectrode device, aimed at providing treatment for chronic pain.
The trial, known as the Lumbar Injectrode Feasibility Evaluation (LIFE) study, has reportedly met all its primary and secondary goals, demonstrating both safety and effectiveness.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
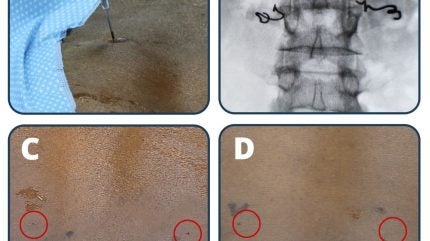
The LIFE study involved ten participants who received the Injectrode, an injectable electrode, for a period of less than 30 days.
Injectrodes were placed near the dorsal rami innervation to the erector spinae and multifidus muscles in the lower back, either unilaterally or bilaterally.
This approach is intended to offer neuromodulatory and rehabilitative treatment for non-radiating, axial, and facetogenic lower back pain.
The placement of the Injectrode was performed under local analgesia, using fluoroscopic visualisation to determine the exact location, and electrical test stimulation to confirm correct placement.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial showcased the feasibility of transcutaneous stimulation with voltages under 30V, both immediately after placement and on day 25+/-3, prior to the device’s removal.
Results from the trial indicate that the Injectrode can effectively deliver electrical neuromodulation at therapeutic levels from an external pulse generator across the skin to the lumbar dorsal rami.
Activation of all parts of the erector spinae and multifidus muscles was confirmed through ultrasound visualisation, patient proprioception, and physician palpation,
Throughout the LIFE study, the Injectrode is claimed to have maintained a safety profile with no serious adverse events reported. Additionally, no post-insertion analgesics or sutures were required for the participants.
Furthermore, the company is preparing to present more detailed findings from the study at upcoming medical conferences.
Neuronoff CEO Manfred Franke said: “It was beautiful to see the trifecta of safety, effectiveness, and aesthetics be demonstrated so clearly. Using an 18 gauge (1.3mm) diameter needle to implant the Injectrode means that a skin puncture remaining after placement is smaller than what is commonly seen during a blood plasma donation.
“This enabled the use of steri-strips instead of sutures at the injection site. While safety, effectiveness, and clinical usability criteria dominated the focus of this study, I believe that future patients and clinicians alike will appreciate that the injection locations had become essentially invisible in under four weeks. For me, aesthetic outcomes of a medical intervention represent a quality of care measure.”





