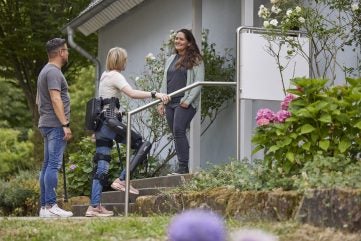
From re-walking to rebranding, ReWalk Robotics has undergone corporate transformation to become Lifeward, as the company turns a corner in its offering to patients with physical limitations or disabilities.
The company began trading under the new Nasdaq stock ticker LFWD on 30 January.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The move reflects recent additions to the company’s portfolio and milestones with the Centers for Medicare & Medicaid Services (CMS) payment.
Speaking to Medical Device Network on what catalysed the rebranding, Lifeward’s CEO Larry Jasinski said: “The opportunities created by the CMS success and our efforts to initiate industry consolidation for growth, efficiency and a faster pathway to profitability.”
In November 2023, Lifeward – at the time still called ReWalk Robotics – made a breakthrough with its personal exoskeleton reimbursement. The CMS set a preliminary payment determination of $94,617.
It came hot on the heels of exoskeletons’ inclusion within the brace benefit category for reimbursement. The addition of exoskeletons to the category followed a rule change released by the CMS on 1 November 2023, with it going into effect on 1 January 2024.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The most significant milestone for the company and the industry with be placement of a price on 1 April 2024 in the CMS Federal Fee schedule for the provision of exoskeletons,” Jasinski told Medical Device Network.
In August 2023, Lifeward completed the acquisition of AlterG for $19m. As a result, Lifeward’s portfolio expanded with AlterG’s anti-gravity systems. Jasinski says that the company is planning new product launches with these devices.
“A new version of the AlterG system is targeted for private practice clinics in Q2.”
Jasinski added that “an FDA submission of a next-generation exoskeleton in Q1 2024 and possibly a clearance and launch in 2024” is also expected.
Lifeward recorded revenue of $4.4m in Q3 2023, up from $0.9m in Q3 2022. The company said its sales of ReWalk systems were stronger in the US compared to Europe.





